Invasive pneumococcal disease in 2024
Annual report for invasive pneumococci for the year 2024
Surveillance of invasive pneumococcal disease in Denmark
Statens Serum Institut (SSI) monitors the occurrence of invasive pneumococcal disease (IPD) in Denmark (Executive Order on Notification of Infectious Diseases). This surveillance has been in place since 2007, when pneumococcal vaccination was first introduced in Denmark. The surveillance enables continuous assessment of the effect of the pneumococcal vaccination programme.
In practice, the surveillance is mainly based ony the clinical microbiology departments sending pneumococcal isolates (Streptococcus pneumoniae) to SSI if the bacterium is detected in normally sterile sites (blood, cerebrospinal fluid, joint fluid, pleural fluid, etc.). SSI also obtains additional information from the Danish Microbiology Database (MiBa), for example in cases where only bacterial DNA could be detected and no viable isolate is available.
The isolates received at SSI are, among other things, serotyped. Serotyping is essential for continuously assessing the suitability of different pneumococcal vaccines, as different vaccines protect against different serotypes. There are more than 100 different pneumococcal serotypes, and available vaccines each protect against up to 23 of the serotypes that most frequently cause disease in humans.
Antibiotic resistance in pneumococci is monitored as part of the DANMAP collaboration. In 2024, resistance to penicillin was 6.4% overall, but different serotypes have different resistance patterns and frequencies.
Overview of invasive pneumococcal disease in 2024
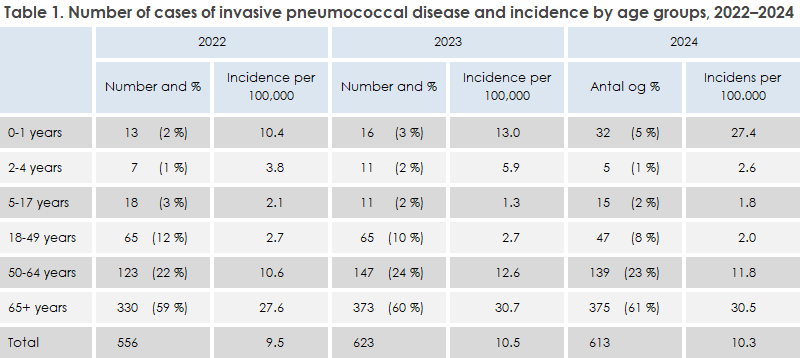
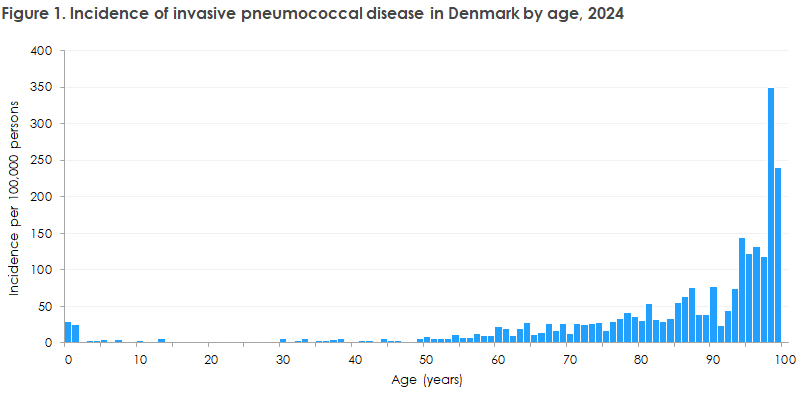
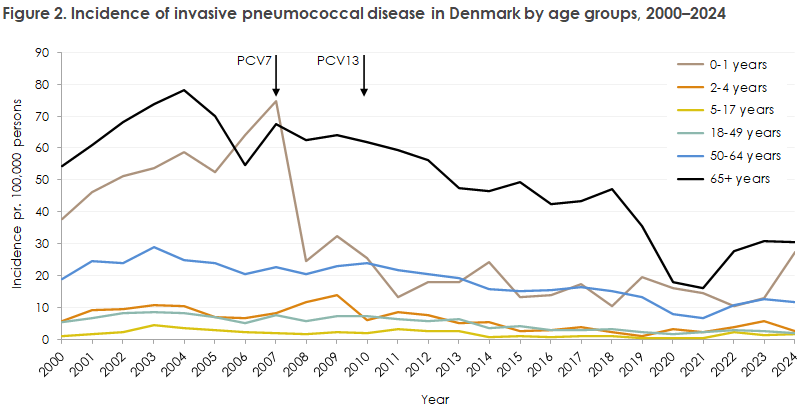
In 2024, 613 cases of invasive pneumococcal disease were registered in Denmark (only Danish citizens are included in the report); 299 cases (49%) were women and 314 (51%) were men. The occurrence in 2024 was very similar to that in the two preceding years, when 623 and 556 cases were registered in 2023 and 2022, respectively. Age distribution and incidence per age group in 2024, as well as corresponding data for 2023 and 2022, can be seen in Table 1. Incidence by age in 2024 is shown in Figure 1, and incidence by age group since 2000 is shown in Figure 2.
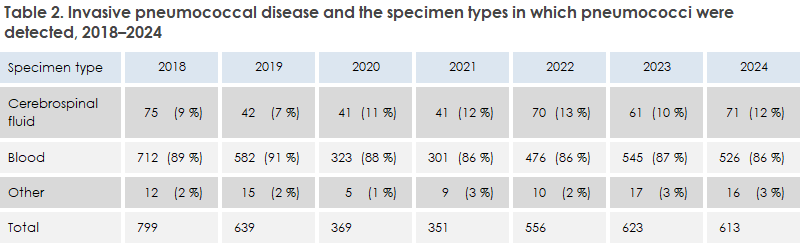
Of the 613 cases of IPD in 2024, 526 were detections of pneumococci in blood (86%), 71 in cerebrospinal fluid (12%), and 16 in other specimens (2.6%; 11 from pleura, 4 from joint fluid, and 1 from peritoneum). The distribution of sample materials was very similar to the distribution in the previous two years, see Table 2. A total of 42 cases were either PCR-detected or the isolate could not be sent to SSI. For these 42 cases, information on serotype is therefore not available.
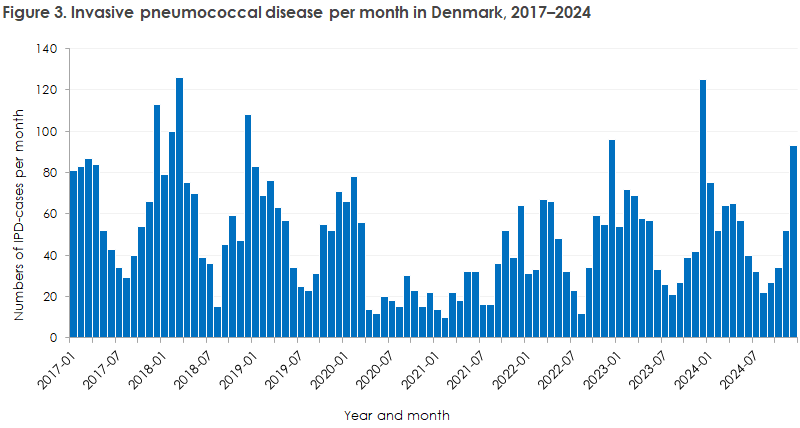
Seasonally, most cases occurred in January–April and in December. IPD typically occurs during the winter period, slightly shifted towards spring. Between 22 (August) and 93 (December) cases of IPD were registered per month in 2024. Monthly case numbers can be seen in Figure 3.
In 2024, 32 cases of IPD among were registered among children under 2 years. For comparison, there were half as many (16) the previous year, and 13 in 2022. The primary reason for the large increase among young children in 2024 was an unusually high occurrence of IPD with serotype 38. Seven of the 32 cases were serotype 38—a serotype that normally occurs rarely in the under-2 age group. In the period 2020–2023, only three cases of IPD in children under 2 years were registered with serotype 38, and up to end of October 2025, no cases have been recorded. Among the remaining 19 cases of serotype 38 IPD in 2024, 17 were older than 60 years, and 2 were aged 30–59 years. Serotype 38 is not included in any available pneumococcal vaccines, and IPD caused by serotype 38 does not differ clinically from IPD caused by other serotypes.
You can read more here: Increasing incidence of serotype 38 invasive pneumococcal disease driven by the ST393 clone among children, Denmark 2022–2024.
Serotypes of pneumococci in invasive pneumococcal disease in 2024
There are some differences in the serotypes that cause IPD when comparing age groups. The serotypes included in the childhood vaccination programme’s vaccines occur rarely among children, but more frequently among older adults. Before the childhood pneumococcal vaccination programme was introduced in 2007, a high proportion of IPD cases among children were caused by the serotypes later included in the vaccines. Among children under 5 years of age, 91% of IPD cases in 2000–2005 were caused by the 13 serotypes included in the Prevenar13 vaccine (PCV13). A similar decline in the proportion of IPD caused by these 13 serotypes has been observed among adults, owing to reduced transmission from vaccinated children.
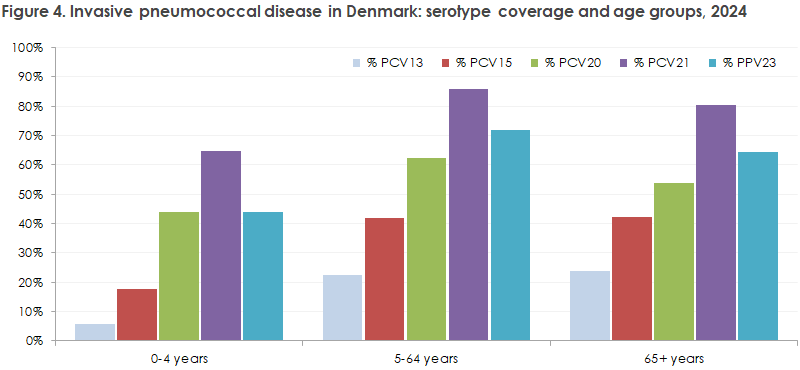
Figure 4 illustrates how many of the 2024 IPD serotypes are covered by the different pneumococcal vaccines. For example, 6% of all IPD cases in children under 5 years were caused by the serotypes included in Prevenar13 (PCV13), and 64% of all IPD cases among persons 65+ years were caused by serotypes included in Pneumovax (PPV23). The figure does not indicate whether individual patients were vaccinated.
Pneumococcal disease can be prevented by vaccination
Childhood vaccination programme
Since 2007, children have been offered vaccination against pneumococci. The first vaccine used protected against seven serotypes (Prevenar) and was replaced in 2010 by a vaccine protecting against 13 serotypes (Prevenar13). From 1 November 2025, the vaccine is being gradually replaced by a vaccine that protects against 15 serotypes (Vaxneuvance), EPI-NEWS 43a/2025.
The vaccination programme has led to a marked decline in the incidence of invasive pneumococcal disease among children under 2 years (Figure 2), and the serotypes included in the childhood vaccine have been greatly reduced among both children and adults. The vaccine is administered at 3, 5, and 12 months of age, and uptake is high. Among children born in 2024, 97% were vaccinated at 3 and 5 months. Not all children born in 2024 had reached 1 year of age at the time of this report, and therefore final uptake of the full programme has not yet been calculated. For earlier birth cohorts, uptake of the 12-month vaccine has been 95–96%.
Some children still develop invasive pneumococcal disease, usually because they were too young to be vaccinated when they became ill, or because they were infected with serotypes not included in the vaccine. In rare cases, IPD may occur with a serotype included in the vaccine—one such case was recorded in 2024 in an otherwise healthy child, representing vaccine failure.
Pneumococcal vaccines for adults
Pneumococcal disease can also be prevented in adults. The risk of severe pneumococcal disease increases with age and is also higher among persons with chronic conditions. In these groups, pneumococcal vaccination may be considered. Several vaccines are approved for adults, each covering a specific set of serotypes. Some serotypes are covered by all vaccines, while others are unique to certain vaccines.
SSI has updated its guideline for pneumococcal vaccination of adults. The guideline states that one may choose between three vaccines (Pneumovax, Prevenar20, and Capvaxive). These vaccines protect against the serotypes that most frequently cause severe disease in adults, as described above.
When choosing a pneumococcal vaccine, several factors may be considered—for example, price, possibility of conditional reimbursement , breadth of serotype coverage, and need for revaccination. Capvaxive and Prevenar20 are given once, while Pneumovax must be given every 6 years.
This report is also described in EPI-NEWS 47/2025.