meningococcal disease 2024
Annual report on meningococcal disease 2024
Statens Serum Institut (SSI) monitors meningococcal disease (MD) in Denmark. The purpose of this surveillance is to enable rapid contact tracing in order to prevent secondary cases among close contacts through treatment with antibiotics and, if relevant, vaccination. It is important that meningococcal isolates or other biological material with findings of meningococcal DNA are submitted to SSI for serogroup determination, thereby allowing the offer of an appropriate vaccine to close contacts.
MD must be of invasive character to be registered in the surveillance system, which includes meningitis, sepsis, and culture or DNA detection of meningococci in blood, cerebrospinal fluid, or material from other normally sterile sites (e.g., joints).
SSI was informed of 26 cases of MD in 2024. Of these, the majority (24 cases, corresponding to 92%) were formally notified by a clinician. A reminder to notify was conducted for two cases, but no notifications were received.
Geography, sex, and age
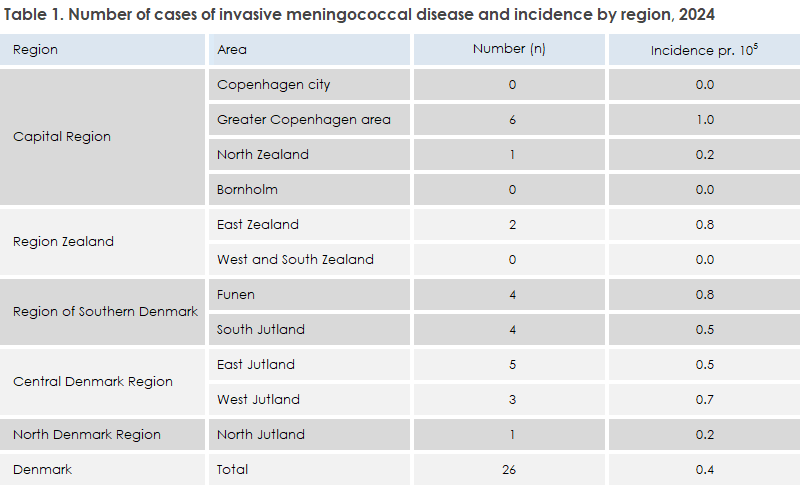
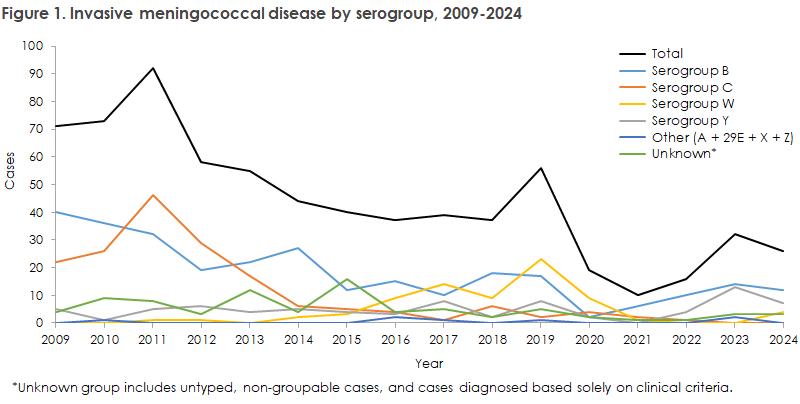
The incidence of MD in 2024 was 0.4 cases per 100,000 inhabitants, which is at the same level as in recent years: 0.5 (2023), 0.3 (2022), 0.2 (2021), and 0.3 (2020). In 2017–2019, the incidence was higher — 0.7, 0.6, and 1.0 respectively (see Figure 1).
Cases in 2024 were evenly distributed across the country, though highest in the Greater Copenhagen area, see Table 1. There were no cases on Bornholm or in Copenhagen City.
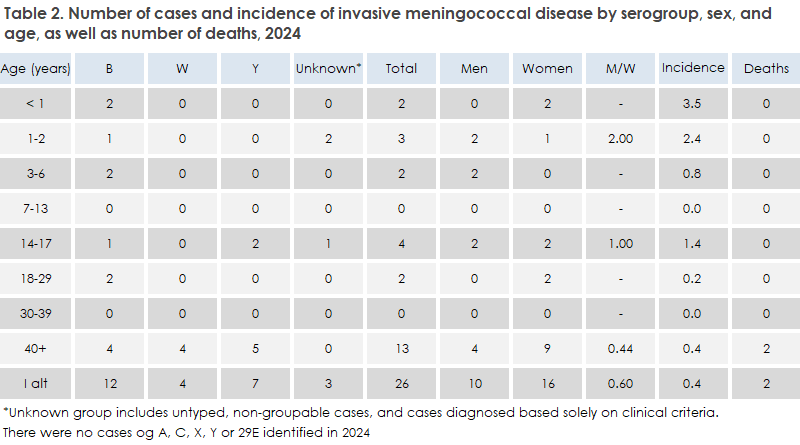
MD occurred in most age groups, see Table 2. The median age was 37 years (range 0–93 years).
The highest incidence was among children under 1 year of age. There were more cases among women than among men.
Diagnosis
Of the 26 cases 7 patients had meningitis, 8 had sepsis, and 9 had both meningitis and sepsis. Two patients were only clinically notified, thus solely based on clinical symptoms consistent with MD.
There were 12 patients with group B (46%), 7 with group Y (27%), and 4 with group W (15%). For 3 patients (12%), the serogroup was not determined. There were no reported cases of serogroups A, C, X, Z, or 29E in 2024.
The presumed country of infection was Denmark for 24 patients (92%). For two patients, information on country of infection was missing as no written notification was received.
In 2022–2023, there had been an increase in serogroup Y, so numbers of serogroups B and Y in 2023 were nearly equal. In 2024, serogroup Y declined again, making serogroup B dominant once more. Group B was detected in almost all age groups, while serogroup Y occurred mainly among persons over 40 years.
Serogroup W has declined since 2019, when a sharp rise occurred and half of all MD cases were group W. In 2022 and 2023, there were no cases of serogroup W, but in 2024, four cases of MD caused by serogroup W were identified.
During 2020–2022, the number of MD cases was low due to the introduction of COVID-19 restrictions in March 2020 (19 cases in 2020, 10 in 2021, 16 in 2022). In 2023, the number of cases doubled compared to 2022, though still below the pre-pandemic level (56 in 2019, 37 in 2018, and 39 in 2017). In 2024, there was again a decrease of nearly 20% compared with 2023 (see Figure 1).
Laboratory investigations
Meningococci were detected by culture in 18 of the 26 cases, and for all these, isolates were sent to SSI for serogroup determination using nucleic acid amplification techniques (NAT) and susceptibility testing. All isolates were susceptible to ceftriaxone, ciprofloxacin, penicillin, and rifampicin. In six cases, meningococcal DNA was detected only by NAT, and for five of these, material was sent to the NSR for serogroup determination. In two cases, no meningococci were detected by laboratory testing, and thus no serogroup or susceptibility data were available.
Mortality and sequelae
Two adult patients (serogroups W and Y) died of MD in 2024 (see Table 2).
Sequelae were registered in three patients who had MD in 2024. Two developed hearing loss, and one developed rheumatoid arthritis. Sequelae are defined as diagnoses consistent with known (from the literature) long-term effects of MD, that have been recorded 14 days to 1 year after the disease date.
Vaccination of close contacts
In cases of suspected or confirmed MD, a consultant physician from the Danish Patient Safety Authority (STPS) determines who qualifies as close contacts and ensures prompt preventive antibiotic treatment. If a vaccine-preventable serogroup (A, C, W, Y, or B) is later identified, STPS sends a letter via e-Boks to the close contacts offering free vaccination against the detected serogroup. The close contact is instructed to contact their general practitioner (GP) for vaccination.
The GP can request the vaccine free of charge by contacting the on-call epidemiologist at SSI.
The vaccine should be administered no later than 4 weeks after exposure.
In 2024, STPS sent letters to 103 close contacts, and a little over half were identified to have received the vaccine. SSI is working to improve surveillance of meningococcal vaccine distribution.
Background Information on Reporting Obligations
MD is classified as a List 1a disease under the Executive Order on the Notification of Infectious Diseases and must therefore be reported both by telephone and in writing to the Danish Patient Safety Authority and SSI (see Disease Surveillance). The notification obligation enables rapid contact tracing to prevent secondary cases through antibiotics and vaccination see Guidelines on Prevention in Cases of Meningococcal Disease. It is crucial that meningococcal isolates or other biological material containing meningococcal DNA are submitted to SSI for serogroup determination, allowing appropriate vaccination of close contacts see Invasive Meningococcal Disease, Post-Exposure Prophylaxis.
In Denmark, MD is clinically notifiable via the Danish Health Data Authority’s Electronic Reporting System (SEI2). Surveillance is carried out jointly by Statens Serum Institut (SSI), Department of Infectious Disease Epidemiology and Prevention, and The Neisseria and Streptococcus Reference Laboratory, which receives isolates and other material from clinical microbiology departments.
The two surveillance systems continuously complement each other.