No 17 - 2016
Annual reports on the danish childhood vaccination programme
Annual reports on the danish childhood vaccination programme
In this week, the WHO celebrates the 11th European Immunization Week. The WHO aims to promote vaccinations as an effective means of prevention against infectious diseases, and the week is marked in various ways in each of the member countries.
In Denmark, the Danish Health Authority, the Danish Medicines Agency and Statens Serum Institut have jointly prepared annual reports on the Danish childhood vaccination programme for 2014 and 2015.
The annual reports describe the vaccination programme and the activities associated with the programme in each year. The annual report is thus designed to serve as a one-stop reference on the childhood vaccination programme.
Henceforth, the annual report will be published every year in April in connection with the European Immunization Week. The annual report is available from the three organisations’ websites.
Overall, the childhood vaccination programme serves its purpose, but there is room for improvements. The 2015 annual report places a special focus on the challenges associated with the decreasing coverage of the HPV vaccine.
Furthermore, 2015 saw quite a lot of debate about measles in Denmark. The debate was triggered by a major measles outbreak in Berlin and a more modest outbreak in Denmark. Even though only a limited number of cases of measles are seen in Denmark, measles outbreaks may also occur in the future as long as the vaccination coverage does not reach the 95% threshold.
The objective of the childhood vaccination programme
Through the childhood vaccination programme, the Danish authorities strive to protect the individual person against a number of diseases. The vaccination programme also serves to prevent infection from spreading in the community and affecting persons who have not received vaccination, either because they are too young or too ill to receive the vaccine.
Many of the infectious diseases that form part of the childhood vaccination programme will run an epidemic course in unvaccinated populations every few years, but the epidemics can be avoided through a high vaccination coverage in the population. For example, the introduction of the pneumococcal vaccine for children has been seen to provide a certain level of protection against pneumococcal disease, not only in children, but in the entire population owing to the general preventive effect of the vaccine.
The childhood vaccination programme will achieve a high coverage only if the population trusts the authorities’ handling of the vaccination programme, and if there is a high level of support for the vaccination programme in the population. Therefore, the Danish health authorities inform parents, day-care institutions, schools and healthcare workers about the authorities’ recommendations and the benefits achieved through vaccination of children.
The information is disseminated through a number of publications, e.g. the folder The Danish childhood vaccination programme (2015) , the guideline Infectious diseases in children and adolescents - A guideline on prevention in day-care institutions, schools, etc. (2013) and through information campaigns.
Additionally, the childhood vaccination programme is monitored closely, and any reported presumed side effects of the vaccines are analysed continuously in Denmark as well as at the European level.
Criteria that need to be met for a vaccine to be included in the childhood vaccination programme
The main factors taken into account in recent years by the Danish Health Authority when considering if a vaccine should be included in the childhood vaccination programme are as follows:
- The severity and prevalence of the condition. For a vaccination to be introduced, the condition needs to have a certain severity and prevalence in order to justify any risk of side effects in otherwise healthy children.
- Broad experience with use of the vaccine in children. The vaccine must have been tested in large groups of children to ensure that the safety and effect of the vaccine are acceptable.
- Beneficial effect versus any side effects. Sufficient documentation must be in place to ensure that the benefits of the disease-preventive and health-promoting effect of the vaccine outweigh the risk of side effects.
- Parental accept. Any new vaccine as well as the overall childhood vaccination programme must be acceptable to the children’s parents.
- Interactions and adaptation to the vaccination programme. It must be ensured that the vaccine can be introduced into the childhood vaccination programme, that the various vaccines will not affect each other negatively (interactions) and that there are no undesired ecological effects (that the micro-organism that is the objective of the vaccine will not simply be replaced by others).
- Overall financial cost-benefit. The introduction of the vaccine shall yield a good trade-off between its costs and benefits.
The severity criterion is given considerable weight when deciding which conditions will form part of the childhood vaccination programme.
Recommendations on vaccination coverage
The more infectious a condition is, the higher a level of immunity is required in the population to ensure that the condition will cause no major outbreaks.
Measles is one of the most infectious childhood conditions and for outbreaks to be avoided the population coverage must therefore be high. The WHO’s objective for measles is a minimum 95% coverage. Therefore, a minimum of 95% of all children should receive both MMR vaccines. Nevertheless, this objective has not been reached for any birth year since the vaccine was introduced into the childhood vaccination programme in 1987, EPI-NEWS 16/15.
For polio, the WHO assesses that a minimum of 90% of all children should receive vaccination, and this objective has been reached for the three first DTaP-IPV/Hib vaccinations since birth year 2009. And transmission of polio has not been observed in Denmark since 1976.
Vaccination coverage in 2015
In 2015, the vaccination coverage of the childhood vaccination programme was as presented in Table 1.
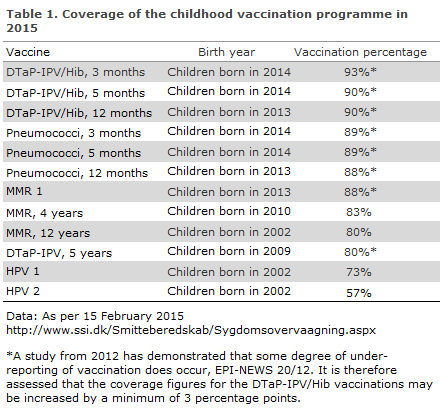
In a study, Statens Serum Institut documents that the calculated vaccination coverage is under-estimated by 3.1 to 4.3 percentage points for the DTaP-IPV booster vaccination, EPI-NEWS 20/12. For certain vaccines, the real vaccination coverage is thus at least 3% higher than the figures presented in Table 1.
The coverage of the Danish childhood vaccination programme remained stable for several vaccines when comparing 2015 with 2014. Thus, the coverage of the DTaP-IPV/Hib, pneumococcal and the first MMR vaccination remained at the level observed in the previous year. Nevertheless, a decrease was observed in the coverage of the second MMR vaccination among 12-year-olds, from 87% in 2014 to 80% in 2015. Similarly, the coverage decreased by 3 percentage points for booster vaccination at 5 years of age.
The most distinct decrease in the coverage of the childhood vaccination programme was seen for the HPV vaccines. In 2014, the coverage of the first vaccination (birth year 2001) was 80% and for the second vaccination coverage was 74%. In 2015, the coverage decreased to 73% and 57%, respectively (birth year 2002).
The coverage of the different vaccinations under the childhood vaccination programme varies between the various parts of Denmark. In general, the coverage is highest in West and North Jutland and lowest in the City of Copenhagen and in North Zealand. The largest difference between areas is seen for the HPV vaccinations, where the difference was 14 and 21 percentage points for the first and second vaccination in 2015. (Figures, charts and maps presenting the vaccination coverage are available at www.ssi.dk/data).
As from 15 November 2015, all physicians have been duty bound to report all vaccinations they administer in the Danish Vaccination Register, EPI-NEWS 45/15.
Reports concerning vaccines given under the childhood vaccination programme, except for the HPV vaccine
In 2015, a 75% increase (to 412 cases) was observed in the number of reports received; 36 of these cases were classified as serious.
The increase was associated with the DTaP-IPV/Hib vaccine in particular. Many of the reports were non-serious cases of formation of granuloma, and only a limited number of the granulomas had occurred in 2015. The increase was probably fuelled by the fact that there was a strong focus on this side effect.
A granuloma is an itchy, persisting lump formation at the injection site. Granuloma may result in increased hair growth, a changed level of pigmentation in or eczema in the skin above the granuloma.
Typically, the granuloma presents 3 months after the vaccination, but it may occur anywhere from weeks to years after vaccination. The occurrence of granuloma following vaccination with aluminium-containing vaccines is a rare and, in most cases, not a severe side effect.
It is believed that the formation of granuloma is caused by aluminium allergy as aluminium allergy can be established in more than 90% of children who have vaccination granuloma. Aluminium allergy in adults is very rare, and there are strong indications that aluminium allergy in children is transitory in most cases. For more information about vaccination granuloma, please read News about side effects, March 2016 (Nyt om Bivirkninger, marts 2016 ).
Reports concerning the HPV vaccine
2015 saw a total of 822 reports concerning presumed side effects of the HPV vaccine, Table 2. A total of 474 were classified as serious.
45% of the presumed side effects occurred in the 9-14-year age group. This age group was vaccinated as part of the childhood vaccination programme.
29% of the side effects occurred in the 19-30-year age group. Some of these women may have been vaccinated in connection with an extra offer of HPV vaccination given in the 2012-2014 period, or they may themselves have covered the vaccination costs.
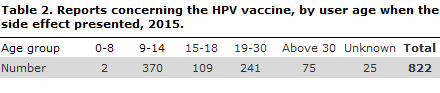
A fourth of all reported presumed side effects concerned symptoms from the nervous system. The most frequent symptoms were headache and dizziness, followed by fainting and difficulty concentrating and focusing attention. Other symptoms were also reported, including, among others, fatigue, pain and malaise. Finally, some of the reported presumed side effects concerned symptoms in joints and muscles, e.g. pain and weakness of the muscles.
Establishing the connection between the presumed side effect and the vaccine is difficult at times as the reports often describe a complex disease picture without unequivocally indicating a specific diagnosis.
There was a considerable rise in the number of reports concerning the HPV vaccine from 2014 to 2015. Nevertheless, only approx. 10% of the reports received in 2015 concerned cases in which the side effects started in 2015, see News about side effects, March 2016 (Nyt om Bivirkninger, marts 2016 ).
In this case, the increase may also have been affected by the attention that the HPV vaccine has attracted in recent years.
The authorities’ initiatives concerning HPV vaccination
2015 brought a strong focus and much attention on the HPV vaccination under the childhood vaccination programme - both in the population and among the authorities. This was due, in part, to the decrease in the coverage of the HPV vaccination, and in part to a considerable increase in the number of reports of presumed side effects to the HPV vaccine, and finally to the massive public debate in the press and on the social media concerning girls who presented with unexplained symptoms.
The healthcare authorities therefore took various initiatives in 2015 in relation to the HPV vaccine: intensified dialogue with stakeholders, recommendations for standardisation of work-up of girls presenting with unexplained symptoms, review of reports and more research in this field.
Additionally, government funds totalling 7 million DKK were allocated from the so-called SATS pulje (Danish government funds) to further research into the possible side effects of HPV vaccines.
Renewed safety assessments of the HPV vaccines
In November 2015, the European Medicines Agency (EMA) published a renewed safety assessment of the HPV vaccines, an assessment that was initiated on Danish initiative. The report concluded that current knowledge does not show that HPV vaccines are associated with the serious side effects of POTS and CRPS. The syndrome complex of POTS, in particular, was the objective of much discussion in Denmark in 2015.
The EMA’s conclusion was based on a thorough review of the published research articles, data from the companies’ clinical trials and reports on presumed side effects from patients and doctors, and on additional data provided by the member countries, including Denmark. The EMA also consulted with a group of leading experts on vaccines, POTS and CRPS, and assessed detailed information received from various patient groups.
By December 2015, the WHO’s Global Advisory Committee on Vaccine Safety (GACVS) also published a statement on the safety of the HPV vaccines. In line with EMA, the GACVS assessed that, based on the existing knowledge, we have no documentation of any safety issues relating to the vaccines that give rise to a change in the use of these vaccines.
Nevertheless, the safety of the vaccines remains under strict monitoring by both the EMA and by the Danish Medicines Agency.
Medical recommendations for the organisation of “single access”
On 1 June 2015, a department in each of the Danish regions was appointed and given responsibility for work-up of persons with unexplained symptoms that had presented in temporal association with the HPV vaccination. By the end of September 2015, Danish Regions stated that the regions had organised these “single access” departments differently. Therefore, Danish Regions recommended that the Danish Health Authority should take an interest in the field by preparing medical recommendations for the efforts made in the departments.
In November 2015, the Danish Health Authority therefore established a broadly based working group. The working group’s medical recommendations for the regions’ work-up efforts were published in February 2016.
Additional relevant research
In the course of 2015, Statens Serum Institut initiated various research projects to enhance our knowledge about HPV vaccination and the groups of girls that report serious presumed side effects to the vaccine and who experience unexplained symptoms, EPI-NEWS 4/16.
One of the projects explores if there are any differences between the group of women who report serious side effects following HPV vaccination and the group that was also vaccinated, but who have reported no side effects. Another project investigates if the consumption of health services prior to HPV vaccination is different in the two groups of girls.
Furthermore, Statens Serum Institut is planning additional studies to determine the causes of lacking HPV vaccination, e.g. the SSI is planning to compare girls who have received the second MMR vaccination and who have also opted out of the HPV vaccination with girls who have received both vaccinations (MMR 2 and HPV vaccination).
Finally, in October 2015, it was decided in connection with the so-called Sats pulje (Danish government funds) agreement for 2016 to allocate 7 million DKK to research into any side effects associated with HPV vaccination for the 2016-19-period.
Special conditions of importance to the childhood vaccination programme in 2016. In 2014, the vaccine Infanrix hexa® was used as a temporary substitute for the DTaP-IPV/Hib vaccine due to a lack of vaccines from Statens Serum Institut, EPI-NEWS 3/14. The vaccine also protects against hepatitis B. In 2015, all children who had initiated hepatitis B vaccination were offered to conclude their vaccination against the condition. In 2014-2015, approx. 11,000 children received hepatitis B vaccination.
By the end of 2015, the offer of free HPV vaccination for women born in the 1993-1997 period was discontinued. This group had been offered free vaccination as part of a so-called catch-up programme.
In the period 5-23 October 2015, the DTaP-IPV/Act-Hib vaccine for primary vaccination was on back order from Statens Serum Institut due to problems related to the production of the vaccine, EPI-NEWS 41/15. Doctors who had Infanrix hexa® in stock from the temporary programme in 2014 were recommended to use this vaccine for vaccinations given at 3 months of age. The children who initiated their vaccination course with Infanrix hexa® were offered to finish their hepatitis B vaccination. Additionally, the DTaP-IPV vaccine for booster vaccination was also temporarily on back order about half way through the year.
Globally, vaccines are now being produced by a limited number of large pharmaceutical corporations. Therefore, production problems in a single company can affect the security of supply worldwide.
In 2015, several European countries have experienced problems in providing the needed whooping cough vaccines for their national childhood vaccination programmes. This is due partly to the fact that several producers have experienced production issues with this type of vaccine, partly to several countries introducing cellular whooping cough vaccines, which increases demand.
The European authorities attempt to counter these vaccine supply interruptions by coordinating the supply of vaccines and the demand generated by the EU.
(The Danish Health Authority, the Danish Medicines Authority and Statens Serum Institut)
Link to previous issues of EPI-NEWS
27 April 2016