No 41 - 2015
The DTaP-IPV/Act-Hib vaccine is out of stock
Prevention of chickenpox and shingles
Follow-up on VZIG dispensing to pregnant women who have become exposed to chickenpox in the 2005-2015 period
The DTaP-IPV/Act-Hib vaccine is out of stock
The DTaP-IPV/Act-Hib vaccine for primary vaccination is currently on back order from Statens Serum Institut (SSI). The SSI assesses that GPs have approx. 30-40,000 doses on stock, and at an average consumption of 15,000 doses a month, we expect that in most areas the demand may therefore be met during the period until the vaccine becomes available again. Nevertheless, uneven distribution may mean that some clinics will deplete their stock before the vaccine is once again available in about 3 weeks' time.
In the present situation characterised by expected and brief unavailability, we recommend that decentral stocks be prioritised so that primary vaccination of 3-month-old infants is prioritised over vaccination of 5-month-olds who, in turn, should be prioritised over 12-month-old children. Thus, unvaccinated children should be prioritised over children who have already received one or more DTaP-IPV/Act-Hib vaccines.
Nevertheless, the lack of DTaP-IPV/Act-Hib should not cause any delay in the administration of Prevenar13 vaccination. If the physician has run out of DTaP-IPV/Act-Hib vaccines, Prevenar13 should still be given at the recommended point in time. This may result in extra visits to the GP. If preferred, the 3rd DTaP-IPV/Act-Hib may be given concurrently with MMR1 at 15 months of age to avoid an extra visit. DTaP-IPV/Act-Hib and Prevenar13 may be given at any mutual interval.
If the physician runs out of primary vaccines in the course of the next 3 weeks, the first DTaP-IPV/Act-Hib vaccination will need to be postponed for the 3-month-old children who are affiliated with the practice. If the physician has Infanrix hexa® on stock with a view to conclude vaccination in the temporary programme, EPI-NEWS 50/14, this vaccine may be employed as primary vaccine instead.
Physicians who order other vaccines will also be informed of the back order situation by mail. If the back order period extends beyond the expected 3 weeks, new information and guidance for health workers will be provided via EPI-NEWS.
The SSI regrets any inconvenience that the back order situation will cause for parents and GPs.
(Statens Serum Institut)
Prevention of chickenpox and shingles
Prevention of chickenpox (varicella) and shingles (herpes zoster) was previously described in EPI-NEWS 5/05, EPI-NEWS 45/05 and EPI-NEWS 33/14. Below follows a joint update and revised guidelines.
Background
Chickenpox and shingles are caused by infection with Varicella Zoster Virus (VZV), a very infectious herpes virus. Chickenpox occurs following primary infection with VZV and presents as general malaise, fever and a characteristic vesicopapular rash on the body and mucous membranes. After the primary infection, the virus remains latent in sensory nerve ganglions; and upon reactivation, it may cause shingles which most frequently present as a painful vesicular skin rash.
The rash is generally unilateral and limited to a single dermatome. Shingles may cause prolonged pain - post-herpetic neuralgia, which may continue for months/years. Reactivation of VZV is associated with decreased cellular immunity, and shingles occur even in persons with a high VZV antibody titer.
Chickenpox is infectious as from 2 days before the rash presents and until all vesicles are crusted over. Transmission is mainly airborne through secretions from airways and aerosols with virus particles from the lesions. The incubation period ranges from 10 to 21 days, most frequently 14 days. Singles rash also contains VZV and is infectious to persons who have not previously had chickenpox, but it usually requires closer contact due to the more limited extension of the rash.
In Denmark like in other areas with a temperate climate, chickenpox is most frequently a mild childhood disease, whereas shingles are more frequently seen in persons >50 years of age. It is expected that 98% of adult Danes have had chickenpox. In adults from tropical climate zones, the share of immune persons may be as low as 30-50%. Within Europe, the share of immune adults also varies as adults from Southern and Eastern Europe have a higher risk of being seronegative than adults from Northern and Western Europe.
Complications to chickenpox arise in approx. 5% of cases and most frequently consist in secondary bacterial skin and airway infections. More serious complications such as virus pneumonia and encephalitis are also seen, albeit rarely. Complications are more frequent in immunosuppressed persons and in adults. Special risk groups also include pregnant women, premature infants and neonates whose mothers have not had chickenpox.
Varicella zoster infection in pregnant women and neonates
Pregnant women who develop chickenpox are believed to be at an increased risk of running a serious course compared with non-pregnant women, particularly a risk of developing virus pneumonia. However, evidence in support of this is limited and based on case reports.
The frequency of pneumonia in pregnant women with chickenpox has previously been reported to be 10-14%, whereas more recent prospective studies report a lower frequency down to 2.5%, which is probably associated with the use of antiviral medicines. The risk of serious virus pneumonia presumably increases with the duration of pregnancy due to the uterus' mechanical impact on the diaphragm. Smoking in the mother or extensive disease, specified as more than 100 vesicles, has also been reported to be risk factors for the development of pneumonia.
The risk for the foetus of a pregnant woman developing chickenpox depends on the gestational age, Table 1. The majority of children who are infected in utero are asymptomatic at birth. Nevertheless, some do have visible chickenpox scars and/or develop shingles in early childhood. The risk that the child is infected with VZV increases with gestational age.
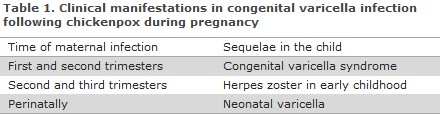
Congenital varicella syndrome (CVS) is seen in children whose mothers develop chickenpox during the first 20 weeks of their pregnancy. The risk is small and has overall been assessed to be approx. 1%. The highest risk (2%) of CVS has been observed in pregnancy week 13-20.. CVS presents as characteristic scar-like skin changes, underdeveloped extremities, malformation of organs, neurologic deficits, etc., and the mortality is high (30%).
Neonatal varicella is seen in children born of mothers who develop chickenpox around their full term. There is a risk of serious disease because the child lacks maternal antibodies, and because its immune system is yet immature. The most serious disease is observed in children whose mothers developed chickenpox from 7 days before to 7 days after giving birth. Neonatal varicella is associated with a mortality of approx. 7%. If the mother develops chickenpox more than 7 days preterm, all neonates will have antibodies and will not be at risk of running a serious disease course.
Preterm children (< 28 weeks) are at a special risk of serious varicella regardless of the mother's immune status as transfer of IgG antibodies primarily occurs in the 3rd trimester. Children with a birth weight below 1 kg are also at risk of lacking maternal antibodies, and they are therefore also at risk of running a serious disease course regardless of the mother's immune status.
Shingles in the mother during pregnancy is not associated with an increased risk for the child as the mother has VZV antibodies that protects the child.
Prevention
Chickenpox and shingles may be prevented through vaccination or treatment with antiviral agents. Chickenpox may also be prevented by treatment with varicella zoster immunoglobulin (VZIG).
Chickenpox vaccination
A live, attenuated vaccine is available for the prevention of chickenpox. The vaccine has been introduced into the childhood vaccination programme of several countries, including the USA, Australia, Germany and Greece. Vaccination consists of 2 doses administered at a minimum interval of 4 weeks and may be given from 9 months of age, EPI-NEWS 25/12.
After a single dose, approx. 85% of the vaccinated children will be protected, increasing to 98% after two doses. Studies from the USA, which has the longest experience with vaccination against chickenpox in its childhood vaccination programme, do not indicate that the immunity wanes over time in vaccinated individuals ; nor are there signs of an increased occurrence of shingles due to reduced natural boosting of immunity.
Approximately 1 month after the vaccination, about 5% develop a transient generalised rash or a rash locally at the vaccination site. Transmission of vaccine virus from the rash to seronegative persons has been described, but is extremely rare; thus, only 10 such cases have been reported in the USA since the introduction of general vaccination in 1995. Serious side effects such as encephalitis or meningitis are extremely rare. For further information on side effects, please see the approved summary of product characteristics.
In Denmark, chickenpox vaccination is used for VZV-seronegative children before organ transplantation and in children with leukaemia after assessment by a specialist. The vaccine is also used in adults who have not had chickenpox, e.g. in seronegative women of childbearing age. Healthy children who have not yet had chickenpox may be vaccinated in order to prevent chickenpox or to concludea vaccination series initiated abroad.
Varicella vaccine can be used as post-exposure prophylaxis if given within 3 days after exposure.
The vaccine may be given to breastfeeding women as the vaccine virus is not excreted into breast milk. The vaccine is contraindicated in pregnant women due to a theoretical risk of infecting the foetus, and pregnancy should be avoided 1 month after vaccination. If a pregnant woman has, nevertheless, been vaccinated inadvertently, there is no indication for abortion. The remaining contraindications for vaccination include persons with serious immune deficiency or with allergies to the ingredients of the drug.
Herpes zoster vaccination
A live attenuated vaccine is available for prevention against shingles and post-herpetic neuralgia. The vaccine is equivalent to the chickenpox vaccine, but holds a 14 times higher antigen content, EPI-NEWS 33/14. This is to ensure an adequate cellular immune response as persons with shingles often already have high antibody titres. Vaccination consists of a single dose and is approved in persons >50 years of age. In a major, multi-centre, double-blinded, placebo-controlled study, the vaccine was shown to prevent approx. 60% of all cases of shingles and post-herpetic neuralgia.
The effect of the vaccine wanes over time, but the need (if any) or the time for booster vaccination has yet to be established. The vaccine's effect in persons who have previously had shingles has not been tested for this group exclusively, but studies have demonstrated that the vaccine is safe for use in this patient group and that it will yield an antibody response comparable to that seen in a zoster-naive population. When vaccinating a patient who has already had shingles, the vaccine should be given after the acute disease course has concluded.
Vaccination is associated with few side effects. Also see EPI-NEWS 33/14 and SSI’s website.
Varicella zoster immunoglobulin
VZIG is a human immunoglobulin derived from donor plasma with high VZV antibody titres. In both healthy and immunosuppressed children who are seronegative and exposed to chickenpox, VZIG has been demonstrated to reduce the seriousness of disease caused by chickenpox or to completely prevent chickenpox. VZIG has not been tested in controlled studies on other patient groups, but it is used to prevent severe disease in neonates whose mothers have not had chickenpox and in seronegative pregnant women who have been exposed to infection. VZIG may also be indicated in immunosuppressed persons. VZIG has no effect once clinical disease has presented.
The VZIG dose is calculated by weight, alternatively by age, depending on the medicinal product, and it is administered intramuscularly. VZIG has a certain effect if given within 4 days of exposure, but may be expected to have some effect up to 10 days after exposure. The half-life is approx. 3 weeks, why repeated administration of VZIG may be necessary in case of repeated exposure.
About 15% of the persons who are treated with VZIG develop a subclinical infection. Serious varicella and death have been observed in patients despite VZIG treatment. Attention should therefore be given to antiviral therapy if signs of development of chickenpox are observed, even after administration of VZIG. Furthermore, it has been shown that VZIG prolongs the incubation period of chickenpox for up to 28 days. A person who has been given VZIG may therefore potentially be infectious in this period. Live vaccines (e.g. against chickenpox or MMR vaccine) should not be given in the 3-month-period following VZIG administration.
The stock of VZIG is small, and the treatment is expensive. Therefore, the indication for VZIG shall always be considered carefully, see Table 2.

Handling of pregnant women who have been exposed to chickenpox
If a pregnant woman, who is not known to have had chickenpox, becomes exposedto VZV, her immune status shall be determined through an antibody test. If she tests negative and a maximum of 10 days have passed from the initial exposure, she could be offered VZIG.
A pregnant woman with a more uncertain exposure, e.g. because her own non-immune child is exposed to chickenpox, the child may be vaccinated against chickenpox and the woman tested for antibodies. If the child develops chickenpox despite vaccination, the woman is offered VZIG if she is seronegative. The parents defray the vaccination costs.
VZIG is given to prevent serious chickenpox disease and pneumonia in the mother; knowledge of the effect in the foetus and development of CVS is limited, but VZIG is expected to also have an effect on the foetus.
If a pregnant woman tests seronegative for VZV, receives VZIG and does not develop chickenpox, vaccination is recommended after birth, but at a minimum of 3 months after VZIG at the earliest. The women herself defrays the vaccination costs.
If a woman develops chickenpox during pregnancy, acyclovir treatment should be considered in consultation with an obstetrician. No teratogenic effect has been observed from acyclovir in pregnant women.
Handling of neonates who have been exposed to chickenpox
See Table 2 for groups of neonates who are at risk of severe chickenpox disease and should be treated with VZIG.
Furthermore, airborne transmission of VZV has been described at hospital wards, and VZIG may be considered for risk patients, at neonatal wards, among others.
For VZV-exposed neonates who are at risk of severe disease, prophylactic acyclovir treatment may be considered in conjunction with VZIG or as a replacement to VZIG if VZIG is delayed or not an option. If a neonate at risk of severe disease develops chickenpox despite these measures, the child should also be treated with acyclovir. This should be done in consultation with a paediatrician.
Handling of immunosuppressed patients who have been exposed to chickenpox
VZIG may be indicated for immunosuppressed patients after specific assessment. In Denmark, VZIG is rarelyused in immunosuppressed children, e.g. children with malignant disorders who are instead treated prophylactically with acyclovir in case of significant exposure.
Commentary
The evidence for treatment of pregnant women and neonates with VZIG is generally limited, as is also the evidence documenting the seriousness of chickenpox during pregnancy. For ethical reasons, no randomised clinical studies have been performed to clarify this topic.
As chickenpox during pregnancy is a rare occurrence in our part of the world, many years of data collection are necessary to describe the outcome in mother and child. Nevertheless, more recent observational studies seem to indicate that chickenpox during pregnancy is less serious than previously assumed. In some countries, e.g. Sweden and Norway, VZIG is not recommended for pregnant women who have become exposed to VZV, and several countries recommend acyclovir for chickenpox in pregnant women.
Acyclovir given as post-exposure prophylaxis may also be considered for both pregnant women and neonates just like it is used in immunosuppressed seronegative children. Nevertheless, evidence for this use is also limited. No teratogenic effect from acyclovir has been observed, but a theoretical risk remains which should be taken into consideration when treating pregnant women. Until more certain evidence becomes available, VZIG continues to be the recommendation in Denmark for seronegative pregnant women who have been exposed to VZV infection.
(I.G. Helmuth, Department of Infectious Disease Epidemiology)
Follow-up on VZIG dispensing to pregnant women who have become exposed to chickenpox in the 2005-2015 period
From December 2005 to March 2015, VZIG was provided for a total of 104 VZV-seronegative pregnant women exposed to chickenpox.
Data from the Danish Civil Registry were available for 101 persons, and 88 answered a questionnaire on their disease course following VZIG treatment. Furthermore, we compared the general characteristics of these women with those of the background population of women of childbearing potential based on age; the data were extracted from Statistics Denmark and the Danish Medical Birth Registry.
Compared with the background population, the women were more frequently immigrants (35% versus 12%) and they were more frequently from Asia, The Middle East and South-East Europe. They were more frequently secundigravida (57% versus 37%, but not older than women of child bearing potential in general (30.6 years versus 30.9 years).
The seasonal variation in dispatching followed the seasonal variation n chickenpox with more dispatches in winter time and in the spring, and fewer in the summer months. A regional difference was observed in the incidence of dispatches, with a lower dispatch rate per female inhabitant of child-bearing potential in Region Zealand and Region of Southern Denmark.
In all, 60% of the women had been exposed through their own child. VZIG was given within 4 days in 57%. Only 7% developed a chickenpox infection, and none reported complications in the form of pneumonia. Three of the 5 women who developed an infection were immigrants, including 2 from Eastern Europe. Only 25% of the women stated having received vaccination against chickenpox after giving birth.
Commentary
It is particularly relevant to enquire about previous chickenpox infection in women of child bearing potential from tropical or subtropical countries, Southern and Eastern Europe. If the woman has not had chickenpox, serological testing is recommended, and possibly chickenpox vaccination before the woman becomes pregnant. Only a small share of the women who were exposed to chickenpox and received VZIG treatment in the course of a 10-year period in Denmark developed chickenpox and none had pneumonia.
It is recommended that women who have received VZIG during their pregnancy and who do not develop chickenpox be offered chickenpox vaccination, e.g. in connection with the 8-week examination. For the vast majority of the treated pregnant women, this examination will be made more than 3 months after their exposure. The women herself defrays the vaccination costs.
C. Jespersen, I.G. Helmuth, T.G. Krause, Department of Infectious Disease Epidemiology
Link to previous issues of EPI-NEWS
7 October 2015