No 33 - 2014
Ebola outbreak in West Africa
New vaccine for meningococcal disease of group B
Vaccine Day at the SSI on 23 September 2014
Vaccine against shingles now available
Ebola outbreak in West Africa
Towards the end of March 2014, an outbreak of ebola haemorrhagic fever was reported from the West African country of Guinea. The outbreak has since then spread to the neighbouring countries of Liberia and Sierra Leone, and within the past few weeks a limited number of cases have also been observed in Nigeria. In this country, however, there are presently no signs of transmission in the community.
According to the World Health Organisation, the WHO, as per 12 August a total of 1,848 cases of ebola had been observed in the four countries. Among these cases, 1,013 were fatal. This is, so far, the largest outbreak of ebola virus, and on 8 August the WHO declared the outbreak a Public Health Emergency of International Concern, PHEIC.
The declaration was motivated by the outbreak being an extraordinary event, the condition serious, the risk of international spreading, and the need for a coordinated international response to limit the spread of the infection. The WHO has issued a series of recommendations to curb the outbreak. See the WHO's recommendations. All of the world's countries have committed themselves to observing these recommendations by adopting the International Health Regulations (IHR).
Ebola haemorrhagic fever is a very serious virus infection, which is associated with a mortality of 50-90%. The condition presents with sudden onset of fever, muscle aches, general fatigue, headache and a sore throat. These symptoms are followed by vomiting, diarrhoea, rash and affected liver and kidney function. In some cases, severe haemorrhaging in various forms is observed, and the disease may progress into multi-organ failure. The disease is only transmitted through contact with bodily fluids and secretions such as blood, vomit, faeces or semen from infected persons. The incubation period is usually 4-10 days, but ranges from 2 to 21 days.
Patients are not infectious until symptom onset, and the majority of cases are therefore seen among healthcare workers who are not wearing protection equipment and among relatives who have had close contact to patients, or among persons who have participated in burial ceremonies, e.g. have prepared a body for burial. No vaccine or specific treatment is available, but experimental medicine in the form of monoclonal antibodies against Zaire ebola virus has been implemented following special permission in individual cases.
This is the first ebola virus outbreak observed in West Africa. The WHO, Médecins Sans Frontières, the Red Cross and other international organisations assist the local healthcare authorities in their efforts to control the outbreak by isolating and treating the diseased and by monitoring any contacts to the diseased persons during the incubation period which lasts up to 21 days.
Furthermore, monitoring of ebola disease and the access to diagnostic tests for ebola have also been boosted in the region, and efforts are made to inform citizens and train healthcare staff. Despite these initiatives, the handling of the outbreak constitutes a considerable challenge due to extensive geographical spreading of the disease cases, substantial cross-border population mobility, fragile healthcare systems with no previous experience of ebola outbreaks, and lack of resources and facilities for isolation and contact tracing.
Additionally, lacking awareness of the disease in the population and lacking trust in the state, international organisations and the existing healthcare system have added to the challenges encountered.
According to the latest risk assessment from the European Centre for Disease Prevention and Control (ECDC), travel-associated cases of ebola may occur in Europe, but the risk of spreading of the virus in Europe is considered to be low. This is owed to the fact that both the infection preparedness measures and healthcare systems of Europe are of a higher standard than those of the affected African countries.
As from 8 August, the Danish Ministry of Foreign Affairs has advised against unnecessary travels to Guinea, Liberia and Sierra Leone. This step was, among others, taken because the healthcare systems in the affected countries are under so much pressure due to the ebola outbreak that treatments offered for other diseases may be affected. Additionally, Liberia as well as Guinea have closed the majority of border crossings to and from the countries, and several airway carriers have suspended all flights to the countries. See the Danish Ministry of Foreign Affairs' travel recommendations.
The WHO has still not introduced any restrictions on travels to the affected countries, and the risk of ebola during travels is considered to be low if travellers observe the precautions to prevent the risk, e.g. by avoiding contact to bodily fluids from diseased persons. The risk is higher when travellers come into contact with health clinics, hospitals, etc. See all recommendations for travel at www ssi.dk.
Generally, all travellers from Africa who present with a fever during or after their travels should see a doctor.
The reason for this is, among others, that such visits will allow the doctor to rule out malaria or other serious infectious diseases. When contacting the doctor, citizens should inform of the symptoms, travel destination and any risk of infection.
On 8 August, the Danish Health and Medicines Authority issued guidelines on the handling of ebola virus disease (EVD). The guidelines are targeted GPs, emergency medical clinics, emergency departments, infectious disease departments and ambulance staff and airports, etc.
For more information about the outbreak, travel recommendations, duty of notification and diagnostics, please see www.ssi.dk.
(T.G. Krause, Department of Infectious Disease Epidemiology)
New vaccine for meningococcal disease of group B
A new vaccine, Bexsero®, has been approved for prevention of infection with Neisseria meningitidis (meningococci) of group B. Henceforth, this vaccine will be offered as post-exposure prophylaxis to persons who have been close contacts with a person with group B meningococcal disease, but it can also be ordered for other patient groups.
Bexsero® is a multicomponent protein vaccine that contains three meningococcal proteins: NHBA (Neisseria heparin-binding antigen), NadA (Neisserial adhesin A) and fHbp (factor H-binding protein) and meningococcal group B outer membrane vesicles.
Vaccine efficacy was estimated by studying the induction of serum bactericidal antibody response to each of the vaccine antigens. It is assessed that Bexsero® may prevent 78% of all cases of group B meningococcal disease in Europe (95% confidence limits 63-90%). The efficacy of Bexsero® has not been evaluated in clinical trials.
Bexsero® is approved for use in infants from two months of age and for adults.
Vaccination programme
The number of doses needed for primary immunisation is age-dependent, see Table 1.
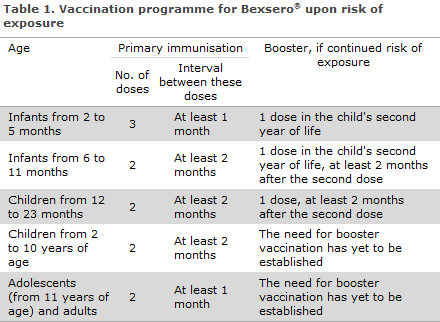
There are no data on the efficacy of the vaccine in adults above 50 years or in patients with chronic medical conditions or immunocompromised persons. It is assessed that the vaccine should also be given as two doses at a minimum interval of 1 month in these groups. The need for booster vaccination has yet to be established.
The most commonly reported adverse reactions in infants and children below the age of 10 years were tenderness and redness around the infection site, fever > 38 °C, irritability, eating disorders, somnolence, unusual crying, diarrhoea, vomiting and rash. Provided Bexsero® was the only vaccine administered, the frequency of fever was in line with that observed at routine childhood vaccinations.
Prophylactic use of paracetamol reduced the occurrence and severity of fever without affecting the immunogenicity of Bexsero®. The most commonly observed local and systemic adverse reactions in adolescents from 11 years of age and in adults were pain at the injection site, malaise, headache, nausea and muscle and joint pains. See the Summary of product characteristics here.
For newly registered pharmaceuticals, all observed or suspected adverse reactions occurring within a two-year period from the marketing date shall be reported to the Danish Health and Medicines Authority. The marketing date was 14 January 2013.
Persons who are to be offered post exposure prophylaxis typically comprise those who have close contacts with a person affected by meningococcal disease, and the Medical Officer of Health decides who such persons are. Vaccine for close contacts can be ordered by contacting the Department of Infectious Disease Epidemiology, Statens Serum Institut.
As Bexsero® is not marketed in Denmark, a special issue permit is needed for the vaccine. Such permit is issued by the Danish Health and Medicines Authority. It is possible to apply for an issue permit for a single patient (individual permit) or for several patients (general permit). If Bexsero® is ordered for other causes, the vaccine shall be ordered directly from the SSI Order Office. In any case, a copy of the issue permit shall be sent to the Order Office at ordre@ssi.dk before the pharmaceutical can be issued.
(C.H. Suppli and P. Valentiner-Branth, Department of Infectious Disease Epidemiology)
Vaccine Day at the SSI on 23 September 2014
The SSI now invites GPs to a training day focusing on the childhood vaccination programme. The day will comprise sessions from the Department of Infectious Disease Epidemiology, the Danish Health and Medicines Authority and the WHO, and there will be ample opportunity to ask questions and share experiences from clinical practice.
See the programme and learn how to sign up
(Department of Infectious Disease Epidemiology)
Vaccine against shingles now available
A vaccine against shingles (Zostavax®) was registered as early as in 2006, EPI-NEWS 26/08, but due to a limited production capacity and prioritisation of some countries, it has only now become available on the Danish market.
The vaccine was approved for prevention of herpes zoster ("zoster" or shingles) and herpes-related post-herpetic neuralgia in patients aged 50 years and above.
The vaccine is supplied as a powder and a solvent for injection as suspension in a pre-filled syringe. Prior to injection, the powder shall be reconstituted in the suspension whereby the vaccine turns into a semi-hazy to translucent, off-white to pale yellow liquid.
The vaccine contains live attenuated varicella zoster virus (Oka/Merck strain) produced in human diploid cells. The vaccine is, in principle, the same as that used for chickenpox, but the antigen contents are approximately 14 times higher.
The vaccination is administered subcutaneously as a single dose (0.65 ml), preferably in the deltoid region. The vaccine may be administered concomitantly with inactivated influenza vaccine as separate injections and at different body sites. The vaccine should not be administered concomitantly with the 23-valent pneumococcal vaccine as concurrent use in a clinical trial resulted in reduced immunogenicity of Zostavax®.
Adverse reactions
In clinical trials, the safety of Zostavax® has been evaluated in more than 32,000 adults. Furthermore, the Summary of Product Characteristics describes adverse reactions reported post-marketing. The frequency of these adverse reactions will not always be known.
The most frequent adverse reactions are general symptoms and injection site reactions such as erythema, pain/soreness, swelling and itching (> 10 %). Headache, local haematoma, warm sensation, and induration are also common adverse reactions (1-10 %). For the remaining adverse reactions, please see the Summary of Product Characteristics, which is available from ssi.dk under "Vaccines".
For information about contraindications and conditions pertaining to fertility, pregnancy and breastfeeding, please see the Summary of Product Characteristics. As Zostavax® should only be given to persons above 50 years of age, as a rule, vaccination of pregnant women will not occur.
Vaccine efficacy
The vaccine's efficacy was established through a range of studies that formed the basis for its registration and a series of follow-up studies, e.g. to assess its long-term efficacy. The vaccine has not been studied in subjects with impaired immunity.
In primary efficacy studies in persons above 60 years of age, the vaccine was 51% efficacious for herpes zoster and 67% efficacious for post-herpetic neuralgia after 3 years of follow-up.
In a follow-up, short-term efficacy study in subjects who had been placebo-vaccinated 4-7 years previously and with an average 1.2-year follow-up period, the estimated vaccine efficacy was 40% for herpes zoster and 60% (non-significant) for post-herpetic neuralgia. The median age in the study population was 73.3 years.
In a follow-up, long-term efficacy study in subjects who had been vaccinated 7-10 years previously in a primary efficacy study and with an average 3.9-year follow-up period, the estimated vaccine efficacy was 21% for herpes zoster and 35% for post-herpetic neuralgia. The median age in the study population was 74.5 years.
Data from follow-up studies thus show that the vaccine's efficacy decreases over time. The need for, or timing of, booster vaccination has not yet been determined.
For patients receiving regular treatment with systemic corticosteroids up to 20 mg/day for a minimum of 2 weeks before vaccination and a minimum of 6 weeks after vaccination, Zostavax® provides a geometric mean 2.3-fold antibody titre increase compared with a 1.1-fold increase in the placebo group, which is at par with the titre increase observed in persons who are not receiving corticosteroid treatment. It is therefore assessed that this group responds adequately to Zostavax® vaccination.
How to store Zostavax
Zostavax® is a live attenuated vaccine and therefore very sensitive to temperature. The vaccine is to be stored at 2-8 °C, and any handling outside of a refrigerator shall be minimised. The vaccine may never be frozen nor reach 25 °C. If possible, the vaccine should be administered immediately after reconstitution and always within 30 minutes. If this time window is exceeded, the vaccine shall be discarded.
How to order
The vaccine will be in stock as from Week 34, and as from 1 September it can be ordered electronically using Form 6 under SSI item no. 69680 Shingles vaccine.
(P.H. Andersen, Department of Infectious Disease Epidemiology, G. Germod, Sales and Business Development)
Link to previous issues of EPI-NEWS
13 August 2014