No 3 - 2014
Vaccination in the temporary vaccination programme
Vaccination in the temporary vaccination programme
This issue of EPI-NEWS is based on "Theme on temporary change of the childhood vaccination programme" published on the SSI website. Substantial parts of the information provided were previously published in EPI-NEWS 49/13, 50/13 and 1-2/14, but we also add new information, e.g. bring the specific service codes to be used. Furthermore, in the last part of this EPI-NEWS, we have tried to answer a number of the most frequently asked questions. Physicians are encouraged to read the full text before initiating the temporary vaccination programme.
Background
Please see EPI-NEWS 42/13, 49/13 and 50/13.
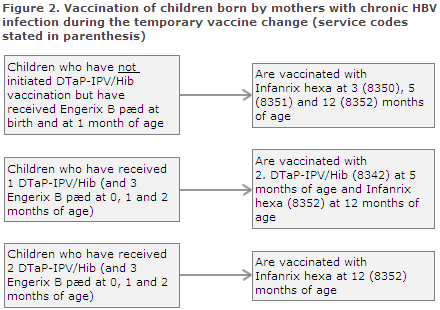
Children initiating their vaccination course in the childhood vaccination programme as from 15 January 2014
Children who initiate their vaccination course under the childhood vaccination programme as from 15 January 2014 are to follow the temporary programme described in Table 1.
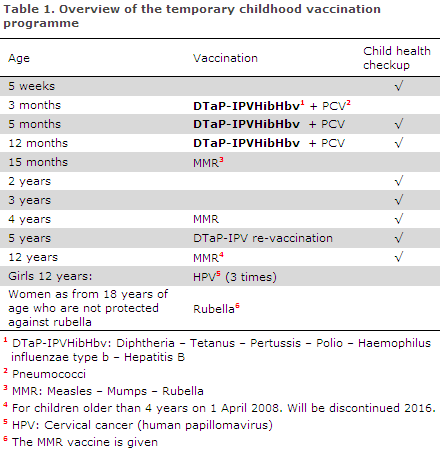
Children who have initiated their vaccination course with the DTaP-IPV/Hib vaccine from the SSI
Children who have initiated their vaccination course with the SSI's DTaP-IPV/Hib vaccine should conclude their course using this vaccine whenever this is possible. The DTaP-IPV/Hib vaccine is still available to order from the SSI. When the vaccine becomes unavailable, the child may be given the DTaP-IPVHibHbv vaccine as a third dose (and, if necessary, also the second dose).
If a child has only received one or two DTaP-IPVHibHbv vaccines, Infanrix hexa®, the child will be offered to conclude the hepatitis B vaccination series free of charge. This will be done by administering the paediatric hepatitis B vaccine Engerix-B pæd®, which may be given in conjunction with other vaccines, e.g. the MMR vaccine, see Figure 1, where the relevant service codes are stated in parenthesis.
Service codes
The monitoring of the vaccination programme's coverage is based on the service codes. Consequently, it is essential that the correct codes are used. Settlements initially go to the National Danish Health Insurance (Sygesikringen), and a validated register is then imported to the Danish Vaccination Register (DVR), which means that the vaccinations will be displayed on the individual child's vaccination card.
The use of correct service codes is also of importance to facilitate monitoring of any adverse reactions and for identification of those children who have been vaccinated against hepatitis B.
The temporary service codes are as follows:
- The Danish Regions and the Danish General Practitioners' Association (PLO) have established temporary codes for the settlement of vaccination with the first (8350), the second (8351) and the third (8352) dose of Infanrix hexa®, Figure 1.
- If a child has received two doses of the SSI's DTaP-IPV/Hib vaccine and is then given Infanrix hexa® in its third dose, code 8352 is used, Figure 1.
- If a child who has received one dose of Infanrix hexa® concludes the hepatitis B vaccination series, the codes used for the second (8353) and the third (8354) dose of Engerix-B pæd® are used.
- If a child who has received two doses of Infanrix hexa® concludes the hepatitis B vaccination series, the code 8354 is used for the third dose of Engerix-B pæd®.
- For the five-year re-vaccination, the previously used booster vaccine should be given (as long as it is on stock locally); alternatively, the polio vaccine (8355) and the DTaP vaccine (8356) are given as two separate injections.
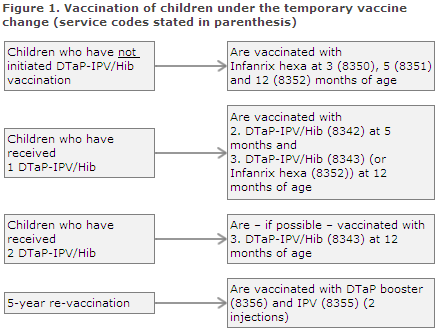
Children born by hepatitis B carrier mothers
Children who were vaccinated at birth and then at one month of age should normally complete their post-exposure prophylaxis with Engerix-B pæd® at 2 and 12 months of age. During the temporary vaccine change, this is done using one or more doses of Infanrix hexa®. Settlement of vaccines given to these children is made using a combination of the service codes normally used for vaccination of children whose mothers are hepatitis B carriers and the new temporary service codes.
Children who have been vaccinated twice with Engerix-B pæd® (at birth and at one month of age) and who have not initiated vaccination with DTaP-IPV/Hib are vaccinated tree times with Infanrix hexa® at 3, 5 and 12 months of age. The codes to be used are 8350, 8351 and 8352; see Figure 2 where the relevant service codes are stated in parenthesis. Giving a total of five vaccines containing hepatitis B entails no risk.
Children who have received three doses of Engerix-B pæd® (at birth and at one and two months of age) and who have received one DTaP-IPV/Hib vaccination (at three months of age) should receive the second DTaP-IPV (code 8342) at five months of age and Infanrix hexa® (code 8352) at 12 months of age, Figure 2.
Children who have received three Engerix-B pæd® (at birth and at one and two months of age) and two DTaP-IPV/Hib vaccinations should receive Infanrix hexa® (code 8352) at 12 months of age, Figure 2.
In situations in which the child's vaccination status does not comply with the above treatment courses, healthcare staff are invited to contact the Consultancy Team, Department of Infectious Disease Epidemiology, by phone (3268 3038) or in writing via epiinfo@ssi.dk.
Children who have initiated their childhood vaccination course abroad in a programme using other vaccines
Children who have been vaccinated abroad and who need to continue their vaccination course under the Danish vaccination programme should be vaccinated in accordance with the guidelines provided in EPI-NEWS 2/11.
Children above 36 months of age who have not yet started their vaccination course under the childhood vaccination programme or who have not yet completed their primary series
No clinical studies on Infanrix hexa® have been performed in children above the age of 3 years. However, considerable experience has been recorded by the countries which use Infanrix hexa® in their national childhood vaccination programmes. In these countries, the vaccine is used for larger children aged 3-10 years of age when adaptation to the vaccination programme is needed.
While the temporary vaccination programme is in force in Denmark, Infanrix hexa® may therefore be used for children aged 3-10 years provided that the guidelines described in EPI-NEWS 2/11 on Hib and minimum intervals are followed.
The Infanrix hexa® vaccine - ordering and provision of the temporary vaccines
The vaccine is provided by the pharmaceutical company GSK and shipped from storage facilities in Romania, why the text on the packing is in Romanian. The vaccine was approved for use in Denmark in 2000, but it has not previously formed part of the Danish childhood vaccination programme. Globally, more than 86 million doses of Infanrix hexa® have been administered. The vaccine is used in many other European countries and in Canada and Australia.
As the vaccine has not previously been used in the Danish childhood vaccination programme, the Danish Health and Medicines Authority has decided that Infanrix hexa® will be subject to an enhanced duty of notification,meaning that doctors are under an obligation to notify all presumed adverse reactions in persons to which they administer the vaccine.
Serious adverse reactions are to be reported to the Danish Health and Medicines Authority no later than 15 days after the doctor has become aware of it, and can be reported through www.meldenbivirkning.dk.
For detailed information about Infanrix hexa and details about how to order and how the temporary vaccines will be provided, please see EPI-NEWS 50/13 and 1-2/14 and the theme and ordering page at the SSI's website.
Questions and answers
Below you will find questions and answers related to the temporary change in the Danish childhood vaccination programme.
What is the difference between DTaP-IPV/Hib and Infanrix hexa®?
The basic DTaP-IPV/Hib vaccine protects against diphtheria, tetanus, pertussis (whooping cough), polio and Haemophilus influenzae B infection. Infanrix hexa® now temporarily forms part of the childhood vaccination programme; it provides protection against the same diseases, but it also protects against hepatitis B.
Why do Danish children not receive hepatitis B vaccination routinely?
Today, the Danish programme does not include hepatitis B vaccination as the risk of infection among Danish children is generally low. In many Central and Southern European countries, hepatitis B forms part of the national vaccination programmes, and this is also recommended globally by the WHO. Denmark has chosen another strategy focusing on vaccination of risk groups, e.g. children of mothers with hepatitis B.
Should children who are not fully vaccinated with Infanrix hexa® pay for the additional doses of Engerix B needed to conclude their hepatitis B vaccination series?
No. If — due to lack of vaccine — it becomes necessary to use Infanrix hexa® to conclude the vaccination of some children whose vaccination course was initiated using the SSI vaccine, these children will subsequently be offered Engerix-B pæd® vaccination free of charge to allow them to complete their hepatitis B vaccination series.
Is the protection against hepatitis B achieved when receiving a single-component vaccine equivalent to that achieved when the vaccine is given as part of the Infanrix hexa®?
Yes. Studies have demonstrated that the level of antibodies in children who have been vaccinated with Infanrix hexa® was no different to that observed in a parallel group who received a single-component hepatitis B vaccine.
How do I settle the temporarily used vaccines with the National Danish Health Insurance (Sygesikringen)?
During the temporary vaccine change, a number of temporary service codes have been introduced; see the sections describing vaccination in the temporary vaccination programme in this issue of EPI-NEWS.
The vaccination card does not hold fields for the Infanrix hexa® vaccine?
The vaccination cards normally handed out to parents will not be re-printed for the temporary vaccine change. In the text field above DTaP-IPV/Hib the person administering the vaccination should write that the Infanrix hexa® vaccine was given, and its batch number should be noted.
Once the primary vaccination series has been completed, for how long will you remain protected?
Once the three vaccinations have been given, the series provides good protection against diphtheria, tetanus, whooping cough and polio until the child reaches 5-6 years of age.
Re-vaccination against diphtheria, tetanus, whooping cough and polio is given at five years of age. After this, the child will be protected against diphtheria and tetanus for another ten years.
Whooping cough protection probably lasts 5-10 years. Polio protection is considered to be life-long.
After three hepatitis B vaccinations, the children are expected to have achieved life-long protection and they are not re-vaccinated.
Why is it that some children are affected by e.g. whooping cough despite receiving the whooping cough vaccination?
Whooping cough protection increases markedly the more of the three vaccine doses the child has received. Therefore, it is important to observe the planned vaccination times at 3, 5 and 12 months of age. The whooping cough component is not 100% effective, and some whooping cough should be expected among fully vaccinated children below the age of two years. Whooping cough is also seen in older fully vaccinated children as protection is limited to a 5-10-year period.
The Patient Information Leaflet stipulates that the vaccine should not be given if the child has experienced problems with its nervous system within the past seven days after having received a vaccine against whooping cough. Does that cause any changes with respect to the previous practice?
No, the previous DTaP-IPV/Hib vaccine also had a precautionary measure for children with progressive neurological conditions. These children should not receive any vaccine containing whooping cough. This also applies if vaccination has previously caused severe reactions.
May a child who is running a fever be vaccinated?
In case of acute disease, vaccination should be postponed. Mild infections such as a common cold and mild fevers up to 38° C do not provide sufficient grounds to postpone vaccination. Children who are possibly developing a disease or who are in the process of being diagnosed should not be vaccinated until the condition is stable and they have been diagnosed.
Is it possible to give half the vaccine dose to avoid adverse events, e.g. in a very small child?
No, the full dose must always be given to achieve a sufficient immune response, i.e. protection. A reduced dose entails considerable risk of rendering the vaccination completely ineffective.
(P.H. Andersen, L.K. Knudsen, P. Valentiner-Branth, Department of Infectious Disease Epidemiology)
Link to previous issues of EPI-NEWS
15 January 2014