No 5a - 2015
Changed recommendations on adaptation to the Danish childhood vaccination programme
Changed recommendations on adaptation to the Danish childhood vaccination programme
In the Danish childhood vaccination programme, the tetanus and polio components have the same strength in the primary and booster vaccine, respectively, whereas the strength of the diphtheria and pertussis components are reduced in the booster vaccine compared to the primary vaccine, to ¼ and ½ of the primary vaccination strength, respectively.
When adapting to the childhood vaccination programme it has previously been a principle that the child should have received primary vaccination with DTaP(-IPV)-containing vaccine with a high titre (diphtheria) antigen, before receiving booster vaccination with a reduced (diphtheria) antigen content.
Nevertheless, experiences from a number of other countries have demonstrated that if primary vaccination is given or concluded with dTap(-IPV)-containing vaccine with a reduced antigen content for one or more antigens (booster vaccine) this will yield an antibody response to pertussis and protection against diphtheria, tetanus and polio in children aged more than 7-10 years of age.
On this background, the Danish recommendations are amended so that children aged 10 years or more, who lack one or more primary vaccinations, are now recommended to initiate or conclude their primary vaccination series with booster vaccine rather than primary vaccine. At the subsequent booster vaccination, the same booster vaccine is employed.
This amendment also influences which vaccines should be used for tetanus prophylaxis after wound injury; thus, these recommendations have also been updated, EPI-NEWS 5b/15.
Diphtheria, tetanus, pertussis and polio
Vaccination programmes are normally based on DTwP/DTaP (Diphtheria-Tetanus-Pertussis, w = whole cell, a = acellular) and polio vaccine (OPV or IPV), which are given concurrently or in combination. The pertussis component may be either whole-cell pertussis vaccine, which remains the most widely used worldwide, or acellular pertussis vaccines, which are now used by most European countries, including Denmark. The two vaccine types are considered equal with respect to the protection provided against pertussis. In other countries, the vaccination programme is often initiated earlier, and frequently more vaccines are given at shorter intervals than is the case in the Danish programme.
When planning the individual child's vaccination programme, only vaccinations for which satisfactory documentary or verbal evidence is available should be taken into account. All vaccine components are considered separately, even if they are given as part of a combined vaccine. Vaccines given before 8 weeks of age are disregarded. The interval between the 2 first vaccinations should preferably be 8 weeks, with a minimum of 4 weeks. If the minimum interval between 2 vaccines has not been observed, the subsequent vaccination should observe the vaccine interval from the latest of the two vaccines given.
When assessing the need for adaptation, the distinction between uncertain and certain vaccination status is maintained as previously.
In case of uncertain vaccination status, it may be necessary to measure diphtheria and tetanus antibodies.
For interpretation of the antibody response, the following guidelines apply:
Antibodies against diphtheria and/or tetanus:
> 1 IU/ml: booster vaccination may wait for 10 years.
> 0.5 IU/ml: booster vaccination may wait for 5 years.
< 0.1 IU/ml: booster vaccination should be given within few years.
< 0.01 IU/ml: the person is unprotected. This may be because the person has never received primary vaccination, or a very long time has passed since the person was last vaccinated.
Uncertain vaccination status
Children below 5 years of age:Children for whom definite information about prior vaccination is lacking are started afresh in the Danish childhood vaccination programme, using the Danish intervals, i.e. 2 months (a minimum of 1 month) between the 1st and 2nd and 7 months (at minimum of 6 months) between the 2nd and 3rd vaccination. In case of substantial local reactions, antibodies against diphtheria and tetanus should be measured 1 month after vaccination.
Children aged 5-9 years:
To determine if the child has received sufficient primary vaccination, a DTaP-IPV/Hib primary vaccine (DiTeKiPol/Act-Hib ) is administered and 1 month hereafter antibodies against diphtheria and tetanus are measured (If the child has turned 6 years old, the Hib component may be omitted, see below).
Antibody levels < 0.1 IU/ml for either diphtheria or tetanus suggest that the child may not have received primary vaccination. In such cases, an additional 2 DTaP-IPV primary vaccines should be given at the stated minimum intervals. Booster vaccination with dTap-IPV vaccine (DiTeKiPol Booster) is given 4-5 years after the latest primary vaccine.
Antibody levels >= 0.1 IU/ml for both diphtheria and tetanus imply that the child has probably received primary vaccination. In such case, the duration of protection depends on the antibody level. For subsequent vaccination, dTap-IPV vaccine is used for booster vaccination.
Children aged 10-17 years:
To determine if the child has received sufficient primary vaccination, a dTap-IPV vaccine for booster vaccination is administered and one month hereafter antibodies against diphtheria and tetanus are measured.
Antibody levels < 0.1 IU/ml for either diphtheria or tetanus suggest that the child may not have received primary vaccination. In this case the recommendation is primary vaccination with an additional 2 dTap-IPV vaccines for booster vaccination at a 6-month interval, as the first dTap-IPV for booster vaccination may be counted as the first primary vaccine. Booster vaccination with dTap-IPV vaccine for booster vaccination is given 4-5 years after the latest primary vaccine.
Antibody levels >= 0.1 IU/ml for both diphtheria and tetanus imply that the child has probably received primary vaccination. In such case, the duration of protection depends on the antibody level. For subsequent vaccination, dTap-IPV vaccine for booster vaccination is used.
Certain vaccination status
The child is vaccinated in accordance with the below recommendations, taking into account the minimum intervals stipulated for each vaccine, Table 1.
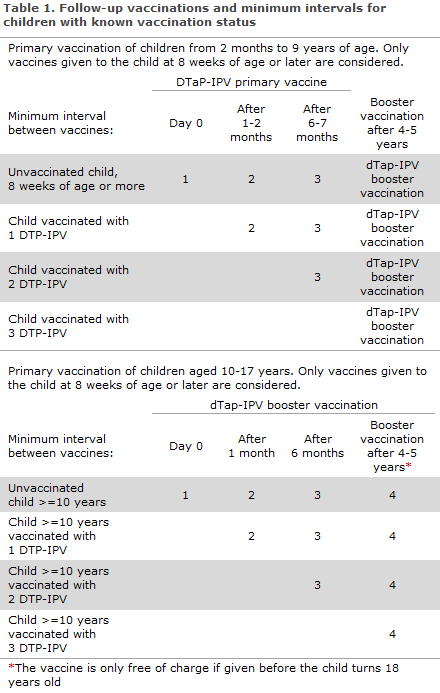
Children from 2 months to 9 years of age:
In children who have previously received DTP/DTP-IPV, only vaccinations given after the age of 8 weeks are considered, see above.
Children who have been vaccinated with:
1 x DTP/DTP-IPV, should receive 2 doses of DTAP-IPV primary vaccine at a minimum interval of 6 months.
2 x DTP/DTP-IPV, given at a minimum interval of 1 month, are recommended 1 dose of DTaP-IPV primary vaccine a minimum of 6 months after the last DT/DTP-IPV.
3 x DTP/DTP-IPV, but where the interval between the 2nd and 3rd vaccine was less than 6 months. One DTaP-IPV primary vaccine shall be given a minimum of 6 months after the 3rd vaccine.
Vaccination with dTap-IPV vaccine for booster vaccination is recommended 4-5 years after the end of primary vaccination, usually at the age of 5 years. Subsequently, dT vaccine (diTeBooster) is recommended every 10 years to ensure permanent protection against tetanus and diphtheria (if wanted, the pertussis protection may also be boosted by using the dTap vaccine for booster vaccination (diTeki Booster) rather than the dT vaccine.
Children below 10 years of age who lack one or more primary vaccines, but who have received a booster vaccine, shall receive the lacking primary vaccines followed by another booster vaccine 4-5 years later.
Children aged 10-17 years:
In children who previously received DTP/DTP-IPV (or dTap/dTap-IPV), vaccinations given as from 8 weeks of age are considered.
Children who have been vaccinated with:
1 x DTP/DTP-IPV, should receive 2 doses of dTap-IPV for booster vaccination at a minimum interval of 6 months.
2 x DTP/DTP-IPV, are recommended 1 dose of dTap-IPV for booster vaccination a minimum of 6 months after the last DTP/DTP-IPV.
3 x DTP/DTP-IPV, but where the interval between the 2nd and 3rd vaccine was less than 6 months. One dTap-IPV vaccine for booster vaccination shall be given a minimum of 6 months after the 3rd vaccine.
Vaccination with dTap-IPV vaccine for booster vaccination is recommended 4-5 years after the end of primary vaccination. Subsequently, dT vaccine for booster vaccination is recommended every 10 years to ensure permanent protection against tetanus and diphtheria (if wanted, the pertussis protection may also be boosted by using the dTap vaccine for booster vaccination rather than the dT vaccine).
Polio
OPV (Oral Polio Vaccine) and IPV (Inactivated Polio Vaccine) are equally efficient with regard to achieving polio immunity.
In children who have previously received polio vaccination, only vaccinations given as from the age of 8 weeks are considered.
Polio vaccine is most frequently given as part of a DTaP-IPV vaccine, either as a primary or as a booster vaccine (depending on the age at the time of vaccination), but may also be given as a mono-component vaccine (IPV).
Children who have been vaccinated with:
1 x polio: Should receive 2 doses of IPV at a minimum interval of 6 months.
2 x polio: Should receive 1 dose of IPV at least 6 months after the previous polio vaccination.
3 x polio, but where the interval between the 2nd and 3rd vaccine was less than 6 months: One additional polio vaccine shall be given a minimum of 6 months after the 3rd vaccine.
Booster vaccination with IPV is recommended 4-5 years after the conclusion of primary vaccination, usually at the age of 5 years.
Hib
In children who have previously received Hib vaccinations, only vaccinations given as from the age of 8 weeks are considered.
As children gradually acquire natural immunity, the number of doses should be reduced with increasing age.
Children under 5 months who have not previously been Hib-vaccinated are given 3 vaccinations at the usual intervals.
Children aged 5-12 months who have not previously been Hib-vaccinated are given 2 vaccinations at a 2-month interval.
Children aged 1-5 years who have not previously been Hib-vaccinated are given only 1 vaccination.
Children aged 6 years or above usually have natural immunity and should not be vaccinated. However, a single Hib vaccination is recommended for unvaccinated splenectomised children until the age of 15 years.
Pneumococci
All children below the age of 2 years are offered vaccination with conjugate pneumococcal vaccine (PCV13). The vaccine may be given as from 2 months of age in a 3-dose programme as the one used in Denmark.
Children who have not turned 1 year old at the first vaccination are recommended 3 vaccines. The minimum intervals are as follows: 1 month between the first and second vaccine, and 2 months between the second and third vaccine (If the child shall also receive primary vaccinations with DTaP-IPV/Hib, these shall be given at a minimum 6-month interval with the 2nd and 3rd DTaP-IPV/Hib).
Children who are older than 1 year at the initial vaccination should be given 2 vaccinations at a minimum interval of 2 months, EPI-NEWS 37a/07.
Unvaccinated children > 2 years are only offered vaccination if they are at a particularly high risk of invasive pneumococcal disease and are therefore entitled to a limited subsidy (Danish: klausuleret tilskud) from the Danish Health and Medicines Authority, EPI-NEWS 40/14.
MMR
Vaccines given before 12 months of age are disregarded. If the child has only been vaccinated against one of the illnesses (usually a measles-only vaccination), the usual 2 MMR vaccinations are given. The minimum interval between 2 MMR vaccinations is 1 month, EPI-NEWS 7/07.
HPV
Girls aged 12 and 13 years are normally offered a 2- or, in some cases, a 3-dose programme, EPI-NEWS 33/14.
The minimum interval in the 2-dose programme is 6 months (shall be concluded within 1 year, otherwise the person shifts to a 3-dose programme).
Girls aged 14-17 years are offered a 3-dose programme.
The minimum intervals of the 3-dose programme are as follows: 1 month between the first and second vaccine, and 3 months between the second and third vaccine. In case of delayed vaccination, the next vaccine should be given as soon as possible, the vaccination sequence should not be reinitiated.
It is recommended to use the same HPV vaccine for the entire vaccination series.
Vaccines that may form part of other countries' vaccination programmes
Hepatitis B
Many countries include hepatitis B in their childhood vaccination programme. In Denmark, a temporary vaccination was in place from 15 January 2014 to 31 December 2014, which included a primary vaccine that also provides protection against hepatitis B (Infanrix hexa®), EPI-NEWS 3/14 and 50/14.
Children who have initiated vaccination against hepatitis B as part of this temporary vaccination programme are offered a total of 3 hepatitis B-containing vaccines (paediatric dose). These are settled using special settlement codes, EPI-NEWS 3/14.
Children who are born in Denmark by an HBV carrier mother shall be offered specific hepatitis B immunoglobulin at birth and a vaccination series with hepatitis B vaccine at birth and after 1, 2 and 12 months, respectively. Furthermore, antibody testing should be performed 2 months after the vaccination series was concluded to document the effect of the post-exposure vaccination programme, EPI-NEWS 4/13.
Children who have initiated hepatitis B vaccination abroad should conclude their vaccination series if they belong to a risk group, i.e. were born by an HBV carrier mother or if there are other persons with chronic hepatitis B in the household, EPI-NEWS 11/13. The vaccinations are settled using special service codes.
It may be expedient to complete the vaccination in other cases in which children have initiated vaccination abroad, but in such cases parents are required to cover the costs themselves.
Meningococci
Some children have received vaccination against one or more types of meningococci. It may be expedient to conclude the vaccination series in such cases, but the parents are required to cover the costs of the vaccines themselves.
Chickenpox
Some children have received vaccination against chickenpox. This vaccine does not form part of the Danish childhood vaccination programme, but is recommended as a 2-dose programme for certain risk groups, EPI-NEWS 5/05 and 25/12. If the child lacks the last vaccination, it may be expedient to conclude the vaccination series, but parents are required to pay for the vaccine themselves.
Tuberculosis
Some children have received BCG vaccination. This vaccine does not form part of the Danish programme, and booster vaccination has no documented effect. Booster vaccination is therefore not recommended.
(The Vaccination Team, Department of Infectious Disease Epidemiology)
Link to previous issues of EPI-NEWS
28 January 2015