No 44/45 - 2020
Update on influenza and pneumococcal vaccination
Update on influenza and pneumococcal vaccination
Influenza vaccine
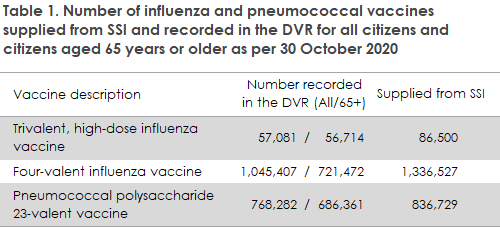
The number of Influenza vaccines acquired corresponds to a 75% vaccination coverage in the at-risk groups, and a SSI supplied a record-high number of influenza vaccines this year. When comparing the number of vaccines supplied and the number of vaccines registered in the Danish Vaccination Register (DVR), considerable differences are apparent, particularly for the four-valent influenza vaccines, Table 1. The difference between the figures may be due to a lag in reporting to DVR and stocks locally that have yet to be used. Currently, a total of approx. 778,000 influenza vaccinations have been registered for persons aged 65 years or more, corresponding to a preliminary vaccination coverage of 67%. For comparison, the final vaccination coverage was 52% in the two preceding seasons. The Danish Health Authority notes that the remaining influenza vaccines should only be used for the included risk groups at this time. See the limits for ordering below.
Ordering from the stock of remaining vaccines
At time of writing, SSI holds a stock of approx. 100,000 doses of four-valent influenza vaccines. SSI will start taking influenza vaccine orders again from the 4th of November 2020.
To ensure a geographically equal distribution of the vaccines, a maximum will be in imposed on orders. Specifically, SSI have just over 3,000 current clients who may order a maximum of 30 vaccines each.
We ask that the maximum number be respected; otherwise, there may not be sufficient vaccines for all clients. Therefore, SSI reserves the right to reduce the number of vaccines ordered.
If any stock remains after the first week, SSI may start taking reorders.
Orders are placed via the SSI’s website: https://bestil.ssi.dk/
Option to return vaccines
SSI clients who hold a minimum of 250 excess influenza vaccine doses, and who assess that they will be unable to administer these vaccines to people covered by the executive order are encouraged to contact the SSI (ordre@ssi.dk) to discuss the option of returning the vaccines.
For SSI to take back vaccines, a declaration is required stating that the vaccines have been refrigerated since they were received along with the electronic temperature data from the refrigerator monitoring system used. Vaccines that SSI assesses can be returned will be picked up by SSI’s refrigerated transport service.
Table 1. Number of influenza and pneumococcal vaccines supplied from SSI and registered in DVR for all citizens and citizens aged 65 years or older as per 30th October 2020
Pneumococcal vaccine
The demand for the pneumococcal vaccine has been high due to the new offer of free pneumococcal vaccination with PPV23 (Pneumovax) for citizens who have turned 65 years old, nursing-home and residential accomodation residents and citizens who are at a particularly high risk of invasive pneumococcal disease, EPI-NEWS 14-16/20 and 23/20. Vaccines were acquired corresponding to a 75% vaccination coverage in the risk groups.
Statens Serum Institut has delivered approx. 837,000 vaccine doses to general practitioners, pharmacies and vaccination clinics, and the vaccine is currently on back order. An additional 260,000 doses were purchased and is expected to arrive no later than February 2021. SSI is in contact with the manufacturer to establish if these vaccines may be delivered earlier.
So far, approx. 768,000 pneumococcal vaccinations with PPV23 have been registered in DVR, Table 1, including approx. 686,000 for persons who have turned 65 years old, corresponding to a preliminary vaccination overage of 59% under the new vaccination scheme. In addition, the estimated vaccination coverage in the target group is approx. 5% based on vaccinations made before the scheme was initiated.
The difference between the number of registered and supplied vaccines may be due to a lag in reporting to DVR and remaining pneumococcal vaccines stocked locally. Pneumococcal vaccines purchased for vaccination under the executive order enforcing the free vaccination offer may not be used for vaccination of anyone not belonging to the mentioned risk groups. Pneumovax for vaccination beyond the limits of the scheme is not expected to be in stock at SSI until the end of 2021. A limited number of doses remain in a blocked stock for use in high-risk patients, and these doses may be ordered by phoning the SSI’s Order Office, Tel.: +45 3268 3111 (Mon-Fri 08.30 a.m. - 3.00 p.m.). If you are unsure whether a patient qualifies for vaccination with vaccines from the blocked stock, feel free to contact the advisory phone service on Tel. +45 3268 3037 (Mon - Fri: 8.30-11.00 a.m., except Wednesdays: 12.30 a.m. - 3.00 p.m.).
Provisions on subsidy for pneumococcal vaccines amended as per 2 November 2020
In connection with the implementation of the national pneumococcal vaccination scheme, the Danish Health Authority has reassessed the person groups who are eligible for a subsidy for the PCV13 (Prevenar 13). The amended provisions mean that, as previously, persons who are at a particularly high risk of invasive pneumococcal disease are eligible for a PCV13 subsidy, whereas the subsidy is no longer available for persons with some chronic conditions, as PCV13 vaccination in addition to PPV23 vaccination are not recommended per se in this group. SSI’s website and corresponding info-graphics have been adjusted to take into account the new provisions on limited subsidy.
(Statens Serum Institut)