No 13 - 2017
Phasing-out of the temporary vaccination programme
Phasing-out of the temporary vaccination programme
As previously announced, the phase-out of the temporary childhood vaccination programme will start on 3 April 2017, EPI-NEW 10/17. Since Week 7/8 2016, the childhood vaccination programme has temporarily employed other vaccines than the standard primary vaccines from Statens Serum Institut (SSI), EPI-NEWS 3/16 and 5/16. This has meant that children receiving the temporary programme were also vaccinated against hepatitis B.
The previously employed primary vaccine (DiTeKiPol/Act-Hib®) is now available again. During the period in which other primary vaccines were used (primarily Hexyon®, but in part of the spring of 2016 also the identical vaccine Hexaxim®, EPI-NEWS 15/16), the SSI’s vaccine production was divested, the final hand-over taking place on 16 January 2017, EPI-NEWS 3/17. The new manufacturer, AJ Vaccines A/S, has also bought the inventory of DiTeKiPol/Act-Hib® that the SSI had already produced and packaged. This means that the primary vaccines provided by the SSI in the coming period will continue to be packed and labelled as an SSI-product (but AJ Vaccines A/S has taken over the marketing authorisation and thus the product liability). The primary vaccine will be packaged as an AJ vaccine only when this surplus stock is exhausted.
To ensure continuity in the childhood vaccination programme, the sale of the SSI’s vaccine production business includes an agreement with the buyer to supply vaccines for the childhood vaccination programme during a 30-month transition period. A bidding round to be initiated in the first half of 2017 will determine which company will subsequently supply vaccines for the programme.
All children who initiate the course of the vaccination programme on 3 April 2017 or later shall therefore receive the DiTeKiPol/Act-Hib® vaccine, and they will not be offered hepatitis B vaccination. The opportunity to conclude the vaccination against hepatitis B will be given to all children who have received a minimum of one hexavalent vaccine as part of the temporary vaccination programme, and who have therefore also initiated a hepatitis B vaccination schedule. The offer will remain in place until the end of March 2018.
It is important to point out that, as previously, no decision has been taken to introduce the hepatitis B vaccination into the childhood vaccination programme. Vaccination against this condition was introduced because the vaccines that were available (Hexyon® and Hexaxim®) as replacements for the SSI vaccine (DiTeKiPol/Act-Hib®) also contained the hepatitis B component. The strategy adopted by Denmark concerning recommendation for vaccination for special risk groups against hepatitis B thus remains in place.
Since 15 November 2015, it has been a legal requirement to record all given vaccines in The Danish Vaccination Register (DVR), EPI-NEWS 45/15. In the transition period from the temporary to the standard vaccination programme, it is, nevertheless, still very important to use the correct service codes.
Children who initiate vaccination under the childhood vaccination programme
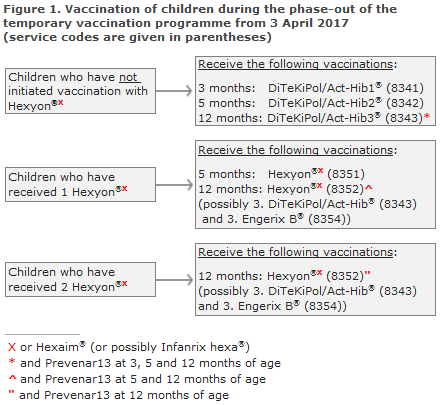
Children who initiate their vaccination course in the childhood vaccination programme on 3 April 2017 or later shall receive primary vaccination with DiTeKiPol/Act-Hib® (code 8341), Figure 1. The hexavalent vaccines (Hexyon®/Hexaxim®/Infanrix hexa®) may not be used as the first primary vaccine after this date. The special settlement code for the first Hexyon® (8350) will be blocked as from 1 May 2017, and settlement of the service will be rejected by the regional authorities after this date.
Children who have received a minimum of one hexavalent vaccine under the temporary vaccination programme
Children who have received a minimum of 1 hexavalent (Hexyon®/Hexaxim®) vaccine and who are therefore partly vaccinated against hepatitis B should conclude their vaccination with 1 or 2 Hexyon® at 5 and/or 12 months of age (code 8351/8352), Figure 1.
Children who have initiated the course of the temporary vaccination programme can only conclude their hepatitis B vaccination until the end of March 2018. Henceforth, the temporary settlement codes will be blocked and settlement of this service will therefore be rejected by the region.
Children who have initiated vaccination with the primary vaccine DiTeKiPol/Act-Hib®, but who concluded their vaccination with one or two hexavalent vaccines
If the lack of vaccine made it been necessary to conclude the vaccination of some children whose vaccination course was initiated using the DiTeKiPol/Act-Hib® with hexavalent vaccine, these children will also receive the offer of Engerix-B® vaccination free of charge to allow them to complete their hepatitis B vaccination series.
Subsequently, Engerix-B® shall be given a minimum of 1 and 6 months, respectively, after the initial hexavalent vaccine containing hepatitis B. Provided the child is not presently at increased risk of hepatitis B, the conclusion of the vaccination may be postponed until the MMR vaccination at 15 months of age and until the 2-year childhood examination, provided that this is possible before the end of March 2018 when the offer expires.
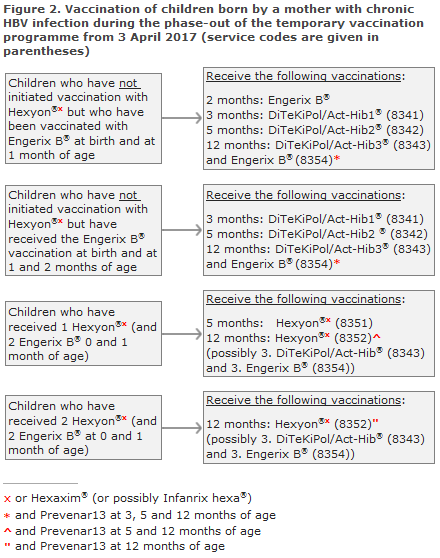
Children born by hepatitis B-positive mothers
Children who have not initiated vaccination with hexavalent vaccine:
The child shall receive Engerix B® (paediatric dose) at birth and at 1 and 2 months of age. Thereafter, DiTeKiPol/Act-Hib® shall be given at 3 and 5 months of age, Figure 2. If Engerix B® at 2 months of age is delayed, it may be given concurrently with the first DiTeKiPol/Act-Hib®. At 12 months of age, the third DiTeKiPol/Act-Hib® and the fourth Engerix B® are given.
All carrier children who have received a minimum of one hexavalent vaccine conclude their vaccination in accordance with the temporary programme:
Children who have received Engerix B® at birth and at 1 month and 1 Hexyon®/Hexaxim® at 3 months of age conclude their vaccination course with two additional hexavalent vaccines at 5 and 12 months of age, respectively, Figure 2.
Children who have received Engerix B® at birth and at 1 month of age and 2 Hexyon®/Hexaxim® at 3 and 5 months of age shall conclude their vaccination course with hexavalent vaccines at 12 months of age, Figure 2.
In situations in which the child's vaccination status does not comply with the above treatment courses, healthcare staff are invited to contact the Consultancy Team, the Department of Infectious Disease Epidemiology and Prevention, by phone (3268 3038) or in writing via epiinfo@ssi.dk.
How to order vaccines
As previously, DiTeKiPol/Act-Hib® is ordered by item number 98949.
For conclusion of initiated hepatitis B vaccination series, it will be possible to order paediatric doses of the hepatitis B vaccine Engerix-B®, SSI Item no. 17606, until the end of March 2018. Orders are placed by phoning the Order Office at 3268 3111.
All other vaccines can be ordered via the SSI's electronic Form 6.
The SSI will not take back vaccines provided for use under the temporary vaccination programme. We therefore urge you not to order more vaccines than you expect to use.
(P.H. Andersen, P. Valentiner-Branth, L.K. Knudsen, Department of Infectious Epidemiology and Prevention, B. Neale, Vaccine Supply and Planning, SYB, The Danish Health Authority)
Link to previous issues of EPI-NEWS
29 March 2017