No 11 - 2015
Hospital-acquired bacteraemia
MMR vaccination for children and adults
ERRATUM
Hospital-acquired bacteraemia
Bacteraemia, also known as bloodstream infection, is a serious infection in which bacteria are found in the blood. Hospital-acquired bacteraemia can now be monitored through the Hospital-Acquired Infections DataBAse (HAIBA), EPI-NEWS 9/15.
HAIBA automatically retrieves patient-administrative data from the National Patient Register, blood culture data from the Danish Microbiology Database (MiBa) and, starting later this year, also data on antimicrobial treatment from the regional medicine modules.
HAIBA makes it possible to monitor the occurrence continuously. HAIBA covers all hospitals and departments in Denmark. Through HAIBA you can monitor local trends and see if, e.g., measures taken have had any impact or if unexpected increases are observed that require further local investigation.
Prior to the introduction of HAIBA, hospital-acquired bacteraemia was only monitored through the prevalence studies. The prevalence studies were performed twice annually as spot checks at selected hospitals and departments. On the basis of the past 5 years' studies, the prevalence of hospital-acquired bacteraemia presumably falls within the 1.1-1.7% range
In contrast to the prevalence studies, HAIBA is based on daily automatic data capture from existing data sources, and the occurrence of bacteraemia will be reported as the number of cases per week, month and year and as incidences.
Background
The mortality from bacteraemia is high, and bacteraemia may occur in connection with infections such as pneumonia or meningitis. Bacteraemia may also occur in connection with infected wounds or surgical interventions; and in patients with immunodeficiency, the patient's own bacteria may invade the blood stream through minor portals of entry in the skin and mucous membranes.
Case definition and classification
To identify patients with hospital-acquired bacteraemia, a case definition was prepared that identifies and classifies bacteraemia episodes.
- Bacteraemia is defined as a patient who has a minimum of one blood culture positive for a pathogenic bacterium.
- If the blood culture was performed in the interval from 48 hours after being admitted to 48 hours after being discharged, the bacteraemia is classified as hospital-acquired.
- A new episode is defined as an infection that has a sample date more than 14 days after the latest positive test. This is independent of the micro-organism in question. Consequently, a finding of bacteraemia with another micro-organism within a period of 14 days will not count as another episode in HAIBA.
The incidence is calculated as the number of bacteraemia episodes per 10,000 at-risk days. At-risk days are calculated as the number of days that pass from 48 hours after admission to 48 hours after discharge, or until bacteraemia presents. Only the first bacteraemia episode of any admission is included in the incidence calculation. If a patient is re-admitted, a new bacteraemia episode may occur.
The HAIBA data are presented at eSundhed, where the case definitions and data model are also described in more detail.
Validation of bacteraemia
The case definition was validated in several ways. An analysis of blood culture practice demonstrated that the differences between the positive rate in blood cultures from the departments of clinical microbiology only varied slightly. As expected, differences were observed in the frequency of the different bacterial aetiologies between the hospitals. These differences may well be explained by differences in the hospitals' patient populations.
In an early phase of the HAIBA project, the case definition was compared with data from the prevalence study of hospitals in the Capital Region of Denmark and Region Zealand. On the basis of these comparisons, the case definition was adjusted.
Furthermore, HAIBA data were compared with data from the North Denmark Bacteremia Research Database (NDBRD). Even though this database is based on individually assessed patient records, there was a considerable overlap between individuals identified as having hospital-acquired bacteraemia in the two databases.
Results
In the 2010-2014-period, HAIBA identified a total of 13,650 cases of bacteraemia, Table 1.
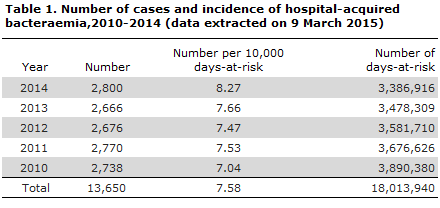
Figure 1 presents the number and incidence of bacteraemia and a combined number for bacteraemia cases and probable bacteraemia cases. In the 2010-2014-period, the number of bacteraemia cases remained stable at around 231 cases per month (median), whereas a slight increase was observed in bacteraemia incidence.
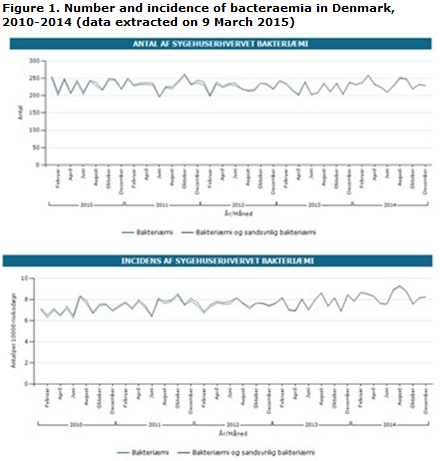
Table 2 presents the number and incidence of bacteraemia cases by region. In the 2010-2014-period, the incidence increased slightly in all regions.
Commentary
HAIBA brings the monitoring of hospital-acquired infections a large step forward, EPI-NEWS 9/15 and 10/15. HAIBA is updated continuously and does not require any active reporting of data as the results are generated on the basis of updated data from MiBa, the patient-administrative systems and, in the future, also from the regional medicine modules. At the launch in 2015, HAIBA includes data from the past 5 years. It is therefore possible to calculate baselines and to study trends for use in the monitoring of bacteraemia for each hospital and department. Generally, a slight increase in incidence is observed for bacteraemia, which may be explained by a decrease in the number of at-risk days owing, among others, to shorter admission periods.
Differences in the number and incidence of bacteraemia in HAIBA shall be interpreted with caution. The number and incidence may vary from one hospital or department to the next, due to, e.g., differences in blood culture practice, antibiotics treatment and the distribution of the patient population, including specific patient groups who are at special risk of bacteraemia.
To take an example, some departments of clinical microbiology report the results of their blood culture per test tube, whereas others report their conclusion for a set of tubes. It is not possible to take these differences into account in HAIBA, and therefore it is not possible to take into account differences in the overall amount of blood that is cultured per patient.
For HAIBA to become a successful tool, it is important that the results are used actively. As mentioned previously, EPI-NEWS 9/15, HAIBA cannot explain why the observed prevalence is as it is, or why changes occur. But dissemination of data through HAIBA may hopefully trigger discussions as to possible explanations and may stimulate studies to better understand the results and to initiate new measures to strengthen the prevention of infections at hospitals.
(S. Gubbels, J. Nielsen, K. Mølbak, M. Voldstedlund, Department of Infectious Disease Epidemiology, B. Kristensen, CEI, K.S. Nielsen, S. Jakobsen, National eHealth Authority, Statens Serum Institut and the regional HAIBA working group)
MMR vaccination for children and adults
The SSI is currently receiving many enquiries from physicians about the possibility of MMR vaccination of adults and about the recommended MMR vaccination schedule for children.
MMR vaccination was introduced into the childhood vaccination programme on 1 January 1987. Therefore, the majority of younger adults up to about 28 years of age have received a minimum of one MMR vaccine. Young adults born in the period from 1974 to 1986, and who are therefore 28-40 years old, were comprised by a catch-up programme including a single MMR vaccination.
The programme's coverage was higher among the younger birth cohorts, EPI-NEWS 12/12. A certain level of opportunistic vaccination activity was seen already in the early 1980s, i.e. before the vaccine was introduced into the childhood vaccination programme, EPI-NEWS 47/82 and 35/84. Furthermore, there was still a considerable number of measles cases in Denmark in the period from 1975 to 1987.
Therefore, the majority of younger adults up to the age of 40 years are typically immune to measles. Nevertheless, about 10% of every birth cohort born as from 1987 and onwards is presumably unprotected. A temporary, free MMR vaccination offer targeting this age group in 2012 only yielded about 2,000 newly vaccinated persons, EPI-NEWS 16/13. Non-immune adults in this age group should receive MMR vaccination, particularly before travelling to areas where measles are in circulation, EPI-NEWS 27a+b/14. Antibody testing ahead of the vaccination is generally not recommended. If you have had one or more of the three conditions, the antibodies will simply neutralise the vaccine component in question.
For all practical purposes, it may be assumed that everyone above 40 years of age have had measles, and there is no general recommendation on MMR vaccination for this age group before travels to areas with measles outbreaks. In cases with specific knowledge of lacking immunity, persons above 40 years may be vaccinated, and there is, in principle, no upper age limit on MMR vaccination.
All adults who have not previously received MMR vaccination or had the disease may be MMR vaccinated, but currently no public payment of the vaccine is in place. When offering MMR vaccines beyond the limits of the childhood vaccination programme, please contact the SSI Order Office by phone. Nevertheless, women of child-bearing age are vaccinated against rubella free of charge if they are not already immune. The offer is provided as an MMR vaccination.
No changes have been made to the recommended schedule for the first and second MMR vaccinations in the childhood vaccination programme. Vaccination is recommended when the child is 15 months and 4 years old, respectively. If general changes are made to the recommended vaccination schedule , the Danish Health and Medicines Authority will inform hereof.
In special circumstances, the first MMR vaccination may be given as from 12 months of age and it will continue to be effective. The vaccine may be given concurrently with the third primary vaccination (3rd DTaP-IPV/Hib and the third Prevenar). Only in case of stays in areas with major outbreaks and therefore considerable risk of infection may the MMR vaccine exceptionally be given as from 9 months of age, but in these cases vaccination shall be repeated when the child is 12 months old or older.
Adverse events may occur following MMR vaccination, but in the overwhelming majority of cases, these are mild and temporary. Some children experience localised pain and swelling at the vaccine injection site, and some run a fever, typically 5-10 days after their vaccination when a rash may also occur that looks much like the real diseases, but is usually milder. The affected child is not infectious.
In adult women, the prevalence of arthritis and arthralgia following vaccination is generally higher than in children (1.2-2%), and the reactions tend to be more marked and of longer duration. This is due to the rubella component of the MMR vaccine. For information about non-common and rare side effects and contraindications, please see the Summary of Product Characteristics of the MMR vaccine (M-M-R-VAXPRO®).
Serious adverse events after MMR vaccinations are uncommon and all occur much more rarely (100-1,000 times) than the complications that are observed after measles disease.
(P.H. Andersen, P. Valentiner-Branth, L.K. Knudsen, Department of Infectious Disease Epidemiology)
ERRATUM
Unfortunately there was a mistake in table 2 in EPI-NEWS 10/15, where the figures for Region North Denmark were erroneous. The correct figures are now available in EPI-NEWS 10/15 at the SSI web page. The editorial team apologize for this mistake.
(Department of Infectious Disease Epidemiology)
Link to previous issues of EPI-NEWS
11 March 2015