No 27a+b - 2018
Vaccination recommendations for foreign travel
Pilgrimages to Mecca
Conclusion of vaccination series with Cervarix® in the childhood vaccination programme
SSI Vaccine Day in Horsens on 25 September 2018
Vaccination recommendations for foreign travel
Statens Serum Institut’s (the SSI) reference group for travel vaccination and malaria prophylaxis regularly revises the SSI’s travel vaccination recommendations. This year brings no changes to the annual summary table, EPI-NEWS 27b/18. That is because the WHO had not published an updated version of the publication International Travel and Health at the EPI-NEWS deadline.
Malaria and stand-by emergency treatment
The reference group has previously assessed that the risk of malaria during travels to a range of areas in Southeast Asia, including Oceania and Central & South America, including the Caribbean, is so low that routine use of medical prophylaxis is not indicated, provided mosquito bite prophylaxis is used diligently, EPI-NEWS 27a/17.
For select travellers who will be staying for prolonged periods of time in areas with a low or mid-level risk and who are willing and a able to use mosquito bite prophylaxis systematically, chemoprophylaxis may be provided for stand-by emergency treatment. This is not relevant for travels lasting less than 7-10 days, corresponding to the malaria incubation period.
It is important to point out that stand-by emergency treatment is not the same as self-therapy. Any stand-by emergency treatment presupposes that the traveller can reach medical assistance within 24 hours and that the physician then detects malaria parasites in the blood. Subsequently, a quality-assured malaria medication may be taken in the form of atovaquone/proguanil 4 tablets daily for 3 days (along with a fatty meal). Stand-by emergency treatment should be given to the traveller only along with written instructions, which, among others, instruct the traveller to bring a thermometer on the journey and to be particularly attentive to fever during the travel and for up to 3 months after returning.
In the tables of EPI-NEWS 27b/18, the stand-by emergency treatment option is indicated by a capital N or a standard n. The SSI website includes a map with areas for which stand-by emergency treatment may be considered in Southeast Asia and South & Central America, respectively.
Influenza
Influenza is the most common vaccine-preventable infection among travellers, approx. 1% per month of travel. Patients who are at increased risk of influenza, EPI-NEWS 38/17, should also be protected during travels abroad, but vaccination of other (healthy) travellers may also be considered. Nevertheless, the seasonal vaccines available in Denmark will not necessarily provide good protection when travelling in the Southern hemisphere, where other influenza types may be dominant. Furthermore, as also seen in Denmark, EPI-NEWS 23-24/18, the efficacy may be reduced due to antigenic drift or increased circulation of the influenza B type which is not included in the year’s trivalent seasonal vaccine. Finally, the effect of the vaccines declines relatively quickly with time. Therefore, persons who do not belong to one of the risk groups should not receive vaccination until 1-2 weeks before their departure.
Japanese Encephalitis (JE)
In case of renewed exposure, anyone below 65 years of age should receive a booster at 12-24 months after the primary 2-dose series, but already after 12 months in case of sustained (local) exposure.
Persons aged 65 years of age or more are also recommended booster vaccination 12 months after the primary series.
Once the first booster has been given, the second booster may be postponed for up to 10 years in persons aged 18-64 years, EPI-NEWS 27a/17. For children and persons above 65 years of age, the duration of protection after the initial booster has not been established.
Data now support that the JE vaccine used (Ixiaro®) may be employed to boost previously administered JE vaccines of other types (Green Cross® or Biken®).
Polio
At a meeting in May 2018, the WHO’s Emergency Committee decided that the international spread of polio (both wild polio type 1 and the circulating vaccine-derived polio viruses, cVDPV) still constitutes a so-called Public Health Emergency of International Concern (PHEIC). It was decided to expand the group of countries from which polio vaccination is required when leaving after stays lasting more than one month in cases where the most recent vaccination was given more than one year previously. In addition to Afghanistan, Pakistan and Nigeria, the group now also includes Somalia (due to circulation of VDPV3).
To ensure a quality-assured vaccine, travellers to these countries who are comprised by the vaccination requirement when leaving are recommended booster vaccination against polio (IPV) before their departure from Denmark and to bring documentation for their vaccination in the international vaccination certificate.
Measles
Measles continues to circulate in many parts of the world, both within and beyond Europe, e.g. in Asia. All travellers should have received measles vaccination. Children can be vaccinated as from 9 months of age; in special cases as from 6 months of age (off-label). If the vaccine is given before 12 months of age, the vaccine is considered to have been administered beyond the childhood vaccination programme and must therefore be repeated twice later (normally at 15 months and 4 years of age). The costs associated with any extra MMR vaccination due to foreign travel are defrayed by the parents.
Adults who are not immune to measles are vaccinated free of charge regardless of their age, EPI-NEWS 13-14/18. Most persons born before 1974 have had measles and are therefore naturally immune.
(C.S. Larsen, Danish Society of Travel Medicine, S. Thybo, Danish Society for Infectious Disease, J. Kurtzhals, Danish Society for Clinical Microbiology, N.E. Møller, Danish College of General Practitioners, L.S. Vestergaard, Danish Society for Tropical Medicine and International Health, K. Gade, The Danish Paediatric Society, P.H. Andersen, A.H. Christiansen, Department of Infectious Epidemiology and Prevention)
Pilgrimages to Mecca
In 2018, the dates for the Hajj are 19-24 August.
Meningococcal disease:
To obtain a visa for Saudi Arabia, anyone doing a pilgrimage must have received the tetravalent vaccine against meningococcal disease of serogroups A+C+W135+Y no later than 10 days prior to entering the country, and the vaccine must have been administered within a 5-year period. Nevertheless, if the international vaccination card does not document that a conjugate vaccine was administered, the validity is only three years.
In Denmark, two four-valent conjugate vaccines are registered for protection against meningococcal disease caused by group A, C, Y or W135; Nimenrix® and Menveo®.
Nimenrix®: The age indication is widened to include neonates as from the age of 6 weeks: The first vaccine may be given as from 6 weeks of age, and the second dose must be given a minimum of 2 months later. A third (booster) dose is recommended at 12 months of age. In all cases, the dose is 0.5 ml. As previously, Nimenrix® may also be used in children ≥ 1 year and in adults.
Menveo® can be used in children aged ≥ 2 years of age and in adults. A single dose is given.
Influenza:
Influenza vaccination is not a requirement but is recommended by the Saudi Arabian authorities, particularly in pregnant women, children below the age of 5 years, elderly persons above 65 years of age and chronically ill persons, see above.
Zika virus infection and dengue fever:
It has been many years since aedes aegypti mosquitoes were last confirmed in the Hajj and Umrah areas, but the mosquito does occur in the surrounding cities. The Ministry of Health of Saudi Arabia recommends that travellers to Hajj and Umrah use mosquito bite prophylaxis 24 hours a day in order to avoid mosquito-borne infections.
Furthermore, it is recommended that travellers observe standard hygiene advice, including:
- avoiding contact with persons suffering from acute infections of the respiratory tract
- maintaining good hand hygiene
- using a mask when staying in densely populated areas
- avoiding close contact to animals, including camels (particularly contact to animal excretions such as saliva and faeces)
- avoiding the ingestion of raw milk and fresh meat.
Persons should see a doctor if they experience severe infections of the respiratory tract (fever with pneumonia and/or difficult breathing) or other severe infectious disease within 14 days after having returned from the Arabian Peninsula.
(A.H. Christiansen, P.H. Andersen, Department of Infectious Disease Epidemiology and Prevention)
Conclusion of vaccination series with Cervarix® in the childhood vaccination programme
When Gardasil®9 was introduced into the childhood vaccination programme as per 1 November 2017, EPI-NEWS 42-43/17, the recommendation was that young women who had initiated their vaccination series with Cervarix® completed their entire series with Cervarix®.
Statens Serum Institut still holds surplus stock of Cervarix®, but the remaining stock will expire on 31 July 2018. Therefore, vaccines can be ordered for young women who can conclude their vaccination course with Cervarix® before the stated date. These vaccines must be ordered by phone to the SSI Order Office and will be delivered as one pack containing 10 vaccines. Any surplus stock of vaccines may be destroyed locally according to standard procedures.
However, physicians may still have vaccines on stock that expire at a later date (batch AHPVA325AF, expiry date 31 October 2019; and batch AHPVA328AD, expiry date 31 December 2019). If you have any of these vaccines on stock, they may be used to conclude vaccination with Cervarix®.
All other young women who have initiated their vaccination series with Cervarix® and who cannot conclude their vaccination series before 1 August 2018 will conclude their vaccination with Gardasil®9 at the intervals recommended for Gardasil®9. Whether or not a person can conclude vaccination as a two- or three-dose programme depends on the person’s age at the first vaccination as well as on the minimum and maximum intervals between vaccinations, Table 1 (in Danish language).
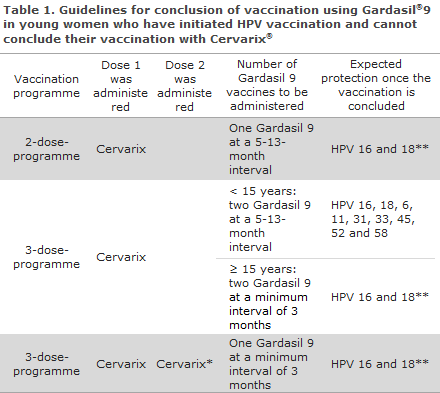
*Vaccination not concluded even though two vaccines were given, see the figure for a description detailing how the vaccines may be given respecting the time intervals (in Danish language).
**Long-term protection may only be expected against the HPV types that form part of the HPV vaccine with which the vaccination series was initiated.
The expected protection against specific serotypes once the vaccination series has been concluded is also presented in Table 1.
For any additional questions, please see Cervical cancer vaccination, (in Danish language).
(L.K. Knudsen, P. Valentiner-Branth, Department of Infectious Disease Epidemiology and Prevention)
SSI Vaccine Day in Horsens on 25 September 2018
Now for the fifth time, the SSI invites GPs and practice staff with a healthcare background related to vaccines and vaccination to attend an instructive and educational day focusing on the childhood vaccination programme. The day will, among others, comprise sessions offered by the General Practitioners’ Association, the Danish Patient Safety Authority and the Department of Infectious Disease Epidemiology and Prevention, and there will be ample opportunity to ask questions and share experiences from clinical practice.
This year, the event will be held at Hotel Scandic Bygholm Park in Horsens.
(Department of Infectious Disease Epidemiology and Prevention)