No 51 - 2016
Serological detection of selected haemoparasites, 2005-2015
Influenza vaccine - new guidelines for reserved stock
Serological detection of selected haemoparasites, 2005-2015
This issue of EPI-NEWS brings a report of the number of patients tested for and cases detected of selected haemoparasitic infections: malaria, schistosomiasis, leishmaniasis, filariasis and trypanosomiasis. The report is based on detection of specific antibodies performed at the Parasitology Laboratory, Statens Serum Institut (SSI), in the period from 2005 to 2015.
The report makes a distinction between “detected cases” and “positive patients” as the number of detected cases is reported annually. Thus, a positive patient can represent more than one case if the patient tested positive several times, possibly in the course of several years. Thus, the number of detected cases will be higher than the number of positive patients.
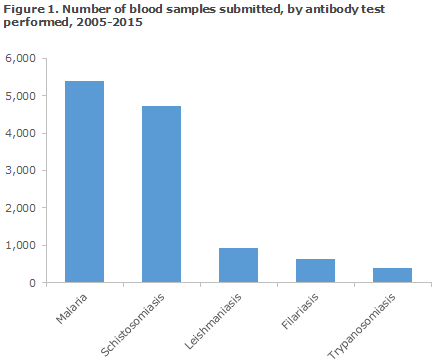
The distribution in the number of blood samples received for serological testing for the five diseases for the 2005-2015 period is presented in Figure 1.
Malaria
Malaria is caused by infection with protozoa of the Plasmodium genus . On suspicion of acute malaria, microscopy or PCR is used, whereas serological testing is relevant, e.g. for screening of blood donors. Blood may thus be tested for Plasmodium-specific antibodies, which supports detection of a previous or ongoing malaria infection.
In the 2005-2015 period, a total of 5,404 samples from 5,105 patients were tested for Plasmodium-specific IgG and IgM antibodies, Figure 2. A total of 261 patients had a minimum of two and a maximum of four blood samples tested. A total of 337 patients tested positive for IgG antibodies, whereas 78 patients tested positive to IgM antibodies. In 23 of the 78 IgM-positive patients, IgG antibodies were not found at the time of the testing.
The share of seropositive patients was 7.1 (360/5,105), which is in line with the 1994-2004 period, when 6.6% of the tested patients were seropositive, EPI-NEWS 44/05.
It is estimated that the majority of the samples had been submitted in connection with screening of potential blood donors.
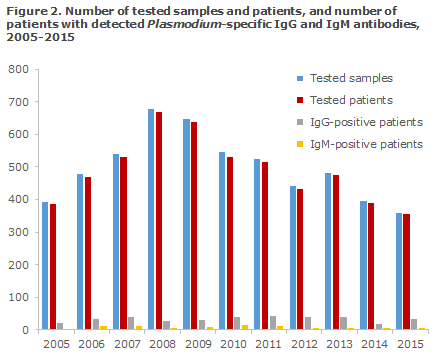
The number of tested samples peaked in 2008 when a total of 677 samples from 670 patients were tested. Since then, the number of samples tested has followed a steadily declining trend; in 2015 the number was 358 samples from 353 patients. Nevertheless, the number of detected cases remained largely unchanged in the course of the period. This may be because the number of blood samples submitted by blood banks for analysis at the SSI has generally been decreasing and because the share of positive samples submitted by blood banks is generally smaller than the share of positive samples from other requestors.
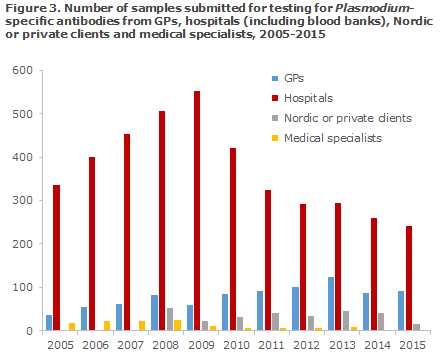
In the course of the period, a trend was seen towards a slight increase in the number of samples submitted by GPs, Figure 3. In contrast hereto, an increase was observed for hospitals (incl. blood banks) until 2009, when the number of tested persons reached 553. Then, a decrease was observed to the 241 samples submitted in 2015.
Schistosomiasis
Schistosomiasis is caused by infection with blood-flukes of the Schistosoma genus . When bathing in freshwater lakes in schistosomiasis-endemic areas, one risks becoming exposed to larvae that penetrate the skin and spread by the blood and lymph to the veins around the urinary bladder or intestine, where they mature into adult unisexual worms. The females excrete eggs that move through the tissue to reach the intestine or urinary bladder and are excreted from there with the faeces or urine.
Owing to their high sensitivity, antibody tests are particularly relevant on suspicion of infection in persons from non-endemic areas, including Danish travellers, as microscopy of urine and faeces will often be negative in patients with “light” infections as are frequently seen in people who have become infected following brief exposure while travelling.
In the entire period, a total of 4,737 samples from 4,027 patients (4,339 cases) were tested for Schistosoma-specific antibodies; 538 patients had more than one blood sample tested in the course of the period. The IFAT analysis is designed to detect antibodies against GAA (gut-associated antigen) and MBA (membrane-bound antigen). GAA antibodies are primarily detected in primary infections, but only rarely in chronic egg carriers. MBA antibodies may both be detected early in the disease course and in the following years. In travellers who are not from an endemic area, a positive GAA and a concurrent negative MBA will underpin the diagnosis of “primary infection”. A negative GAA and a concurrent positive MBA in ethnic Danes is typically not diagnostic, but in immigrants from endemic areas it is often a sign of previous infection. For GAA as well as MBA, a titre corresponding to 1:16 is considered the threshold value. In the present report, a titre value of 1:16 is considered negative.
In all, 668 (16.6%) of the tested patients had antibodies against either GAA and/or MBA in the period, distributed on 767 cases, Table 1. Generally, in the course of the entire period, more tested positive to GAA than to MBA. In the 2009-2010 period, more than every fourth patient tested positive to GAA and/or MBA. In this exact period, a large group of Danish travellers was tested following relevant exposure through river rafting and bathing in the Nile (Uganda) in July of 2009. These persons were followed serologically as a cohort, and approx. one third of the persons seroconverted within the next six months; the majority of the seropositives developed antibodies against GAA, but in a limited number of positive patients, only antibodies against MBA were detected. Considerable variability was seen with respect to the time of seroconversion: from weeks to up to nearly 6 months, which is important to take into consideration when planning the time of sampling and in the differential diagnostic considerations.
It should be mentioned that patients who have become exposed to avian schistosomes, Trichobilharzia, which may cause “swimmer's itch”, e.g. following bathing in Danish lakes, may have developed antibodies that cross-react, primarily with MBA.
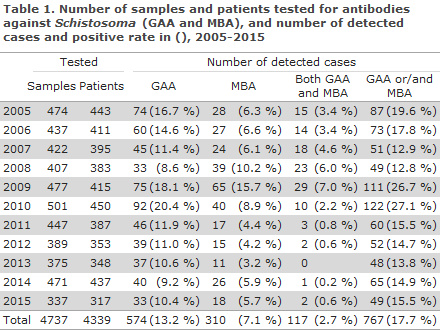
A considerable increase was seen in the share of detected cases from 9.9% in the 1994-2004 period to 17.7% in the 2005-2015 period.
Leishmaniasis
Leishmaniasis is caused by infection with unicellular parasites of the Leishmania genus , which are transmitted through sand fly bites.
Three main types of leishmaniasis exist; visceral, cutaneous and muco-cutaneous leishmaniasis. Leishmania-specific antibodies may usually be detected in patients who have leishmaniasis, except for the cutaneous form for which antibodies may not always be detected.
At the SSI, an immunofluorescence antibody test (IFAT) with titration of antibodies against different life-cycle stages (amastigotes and promastigotes) of Leishmania tropica is performed. A titre of 1:160 or above is considered positive, whereas a titre of 1:80 is considered possibly positive (threshold value). In this report, a sample with a titre of 1:80 is included as positive (detected case).
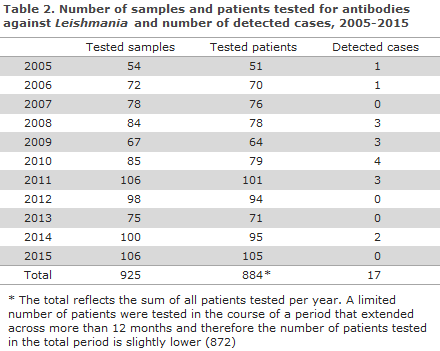
In the 2005-2015 period, a total of 925 samples from 872 patients were tested for Leishmania-specific antibodies, Table 2. The number of tested patients per year in the period ranged from 51 (2005) to 105 (2015). Thus, the number of tested patients for the entire period was 21% higher than that recorded for the 1994-2004 period, EPI-NEWS 44/05. The number of detected cases for the entire period was 17, distributed on 16 patients. Overall, 1.8% of the persons tested positive. The share of test-positive patients in the period was substantially lower than in the previous 11 years, when a positive rate of 7.2% was recorded, EPI-NEWS 44/05.
It is important to underline that these results are only based on serology and that the data therefore do not reflect the full scope of leishmaniasis detected in Denmark. In 2009, the SSI introduced a method for the detection of Leishmania, and the serological data should be considered in conjunction with the recently published report on the number of cases of leishmaniasis detected by PCR in the period from 2009 to October 2016, EPI-NEWS 44/16. It should be mentioned that biopsy material is often submitted from a patient for PCR testing without an enclosed blood sample for antibody determination.
Filariasis
Filariasis comprises a group of diseases that are all caused by infection with closely related microscopic filarial round worms that are all transmitted by mosquitoes or midges. The different forms of filariasis are found in specific geographical areas; for travellers, the most important areas are located in South-East Asia, the Pacific and in Africa, particularly West, East and Central Africa. Symptoms typically only present many months after exposure. Antibody testing is used on suspicion of infection following relevant exposure and may be used for microfilaraemic filariasis (e.g. lymphoid filariasis) as well as for amicrofilaraemic filariasis (e.g. dirofilariasis).
In the 2005-2015 period, a total of 642 samples from 586 patients were tested for filarial-specific antibodies, Table 3. The number of tested patients per year ranged from 35 to 76. It was observed that approx. 10% of the patients with a positive screening test (anti-Dirofilarial IgG antibodies) tested positive to IgG4 antibodies. If IgG4 is detected, it is likely that the infection is productive, i.e. microfilaria can be found in the blood.
The remainder of the screening-positive persons had antibodies that probably represented an older (unproductive) infection - infection with Dirofilaria repens - which does not cause microfilaraemic filariasis in humans (see below), or cross reaction with another nematode. Among patients with a current infection, a minimum of two ethnic Danes were found of whom one tested positive in 2010, 2011 and 2015, but for whom no travel history was available. For the second patient, filarial exposure had probably occurred during a journey to Cameroun. The rest of the IgG4-positive persons had African-sounding names.
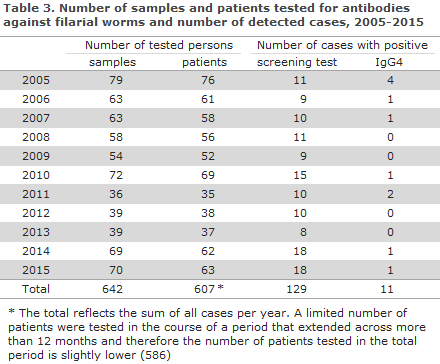
The share of screening-positive patients was 21.3%, whereas only 1.8% of all patients tested positive to current, productive infection. . Even though cross reaction and other methods may explain, in part, this relatively high positive rate, it is also likely that part of the positive screening results may be accounted for by infection with Dirofilaria species that are found in most parts of the world and which may also be transferred by mosquitoes. The Dirofilarial microfilaraemia that is typically seen in dogs, cats and similar animals is not seen in humans, who may, nevertheless, develop subcutaneous nodules, corresponding to the migrating larvae/adult worms.
Trypanosomiasis (African sleeping sickness or Chagas disease)
Trypanosomiasis may be caused by several species of Trypanosoma, including Trypanosoma brucei, which can cause African sleeping sickness, and T. cruzi, that may cause Chagas disease. T. brucei is found in Africa, whereas T. cruzi occurs in South and Central America. T. brucei is further divided into T. brucei gambiense and T. brucei rhodesiense, among which the latter runs the more aggressive disease course.
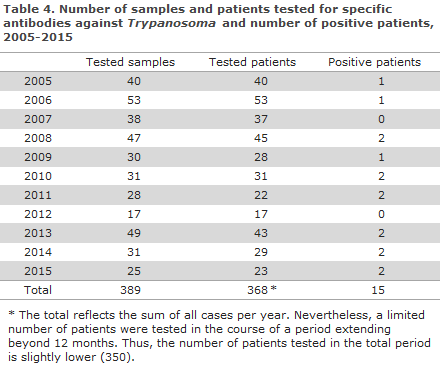
In the 2005-2015 period, a total of 389 samples from 350 patients (368 annual cases) were tested for Trypanosoma antibodies. In this period, a total of 14 persons tested positive (one patient tested positive in two different years; therefore the number of positive patients in Table 4 is 15 in all).
The number of submitted samples ranged from 17 to 53, but no specific trend was observed for the submitted samples over time; nor in relation to the number of positive patients.
Commentary
The current report generally represents nationwide data. Nevertheless, serological diagnostics for the mentioned parasites are also to a limited extent performed at other laboratories of clinical microbiology apart from the SSI. Furthermore, it cannot be excluded that some samples are sent for antibody testing in laboratories abroad, which may, in part, explain the reduction in the number of samples submitted to the SSI from hospitals.
The total number of blood samples submitted for testing for one or more of the five diseases in the entire period was 12,097. Among the described tropical diseases, the conditions most frequently tested for were malaria and schistosomiasis, Figure 1.
The positive rates for the five antibody tests varied somewhat. At one end of the spectrum, the positive rate for the leishmaniasis antibody test was 1.8%. In conjunction with the moderate number of tested samples, this may indicate that the antibody test is not the preferred test for detection of leishmaniasis in Denmark, or that the test is mainly used as a “test of exclusion”. Furthermore, it is estimated that more than half of the samples submitted to the SSI for leishmaniasis testing are submitted mainly for PCR testing on suspicion of cutaneous leishmaniasis for which a negative antibody test will not exclude infection, EPI-NEWS 44/16.
At the other end of the spectrum, 16.6% of the patients tested by the schistosomiasis antibody test were positive, which represents a considerable increase compared with the previous 10-year period. This may be due to more travellers being exposed through bathing in risk areas and to increased attention to subsequent testing of patients.
The five described parasitic infections may be difficult to detect by microscopy or PCR alone, either because it is hard to do the sampling and/or because the infections in question are often only mild or latent.
The advantage of antibody testing is that these tests are often the most simple and also the most sensitive methods for detection of parasitic infections in travellers who have returned from the tropics, including blood and organ donors.
For some of the diseases, e.g. schistosomiasis, it is important for the diagnostic considerations to be aware that antibodies in some patients can only be detected up to 6 months after exposure. The interpretation of antibody results for the mentioned parasites can occasionally be difficult, and factors like relevant exposure (including travel history and recollection of insect bites), clinical findings, symptoms, image diagnostics, possible cross reactions and results from other microbiological and other laboratory tests should be meticulously included in the interpretation of the antibody results.
(C.R. Stensvold, H.V. Nielsen, The Laboratory of Parasitology, Microbiology and Infection Control, L. S. Vestergaard, Department of Infectious Disease Epidemiology)
Influenza vaccine - new guidelines for reserved stock
In collaboration with the Danish Health Authority, it has been decided to use the small surplus stock of 1,500 vaccines, which had otherwise been reserved for vaccination of pregnant women, for vaccination of the remaining risk groups that are comprised by Executive Order of 22 September 2016 on free influenza vaccination to select population groups.
These vaccines will be dispensed on the basis of specific medical indication by telephone contact to the SSI Order Office.
Please note that the Institute does not send out vaccines in week 52, i.e. between Christmas and New Year's Eve.
(Statens Serum Institut)
Merry Christmas & Happy New Year
Unless special circumstances arise, EPI-NEWS will not be published until week 2 , 2017. The editorial team wishes everyone a merry Christmas and a happy New Year.
(Department of Infectious Disease Epidemiology)
Link to previous issues of EPI-NEWS
21 December 2016