No 38 - 2015
Meningococcal disease 2014
Purulent meningitis 2014
New guidelines from the Danish Health and Medicines Authority on the handling of HUS and VTEC
Meningococcal disease 2014
In Denmark, invasive meningococcal disease (MD) is monitored via the clinical notification system (Form 1515) and by the Neisseria and Streptococcal Reference Laboratory, which receives meningococcal isolates from the departments of clinical microbiology.
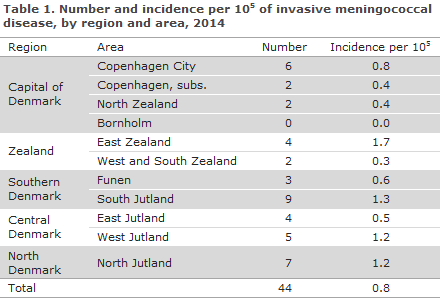
In 2014, the Department of Infectious Disease Epidemiology received a total of 44 notifications concerning patients with MD. A reminder had to be sent out for the notification only in one case. Table 1 shows the distribution by area.
Diagnosis
Among the 44 patients, 14 had meningitis, 10 septicaemia and 19 both meningitis and septicaemia, while one patient had pleural empyema with meningococci.
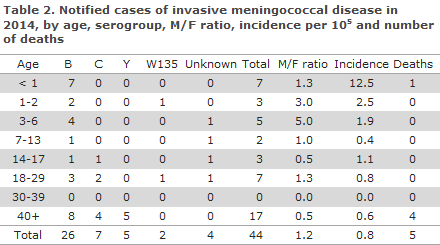
In all, 26 (59%) cases of MD of group B were observed, 7 (16%) group C, 5 (11%) group Y and 2 (5%) group W135. See Table 2, which also presents the age distribution. No cases of group A or X were notified. In 4 (9%) cases, the serogroup was unknown.
Diagnostics
In 34 (77%) of the notified cases, meningococci were detected by culture. Seven cases were detected by PCR, either alone or in combination with microscopy; 2 cases were diagnosed by meningococcal antibody test; and in 1 case the diagnosis was based exclusively on clinical observations. A total of 42 cases were presumably infected in Denmark, and in 2 cases the country of infection was unknown (1 case of group Y and 1 case for which the group was unknown).
Risk factors preceding the condition
In 20 of the 44 cases of MD, an underlying disease and/or predisposition was recorded in the patients: Six had cancer, 1 was splenectomised, 1 had a dural defect, 3 had an infection prior to MD (2 with pneumonia and 1 with acute otitis media), and 9 had various chronic diseases (heart/lung disease, liver disease, endocrinological or neurologic conditions).
Fatality and sequelae from the disease
Five patients died due to MD. Four of these had group B meningococci, 1 had group C. One of the patients with group B meningococci was a premature infant with respiratory distress syndrome and sepsis. The remaining 4 fatalities were >65 years old and only 1 did not have an underlying disease and/or predisposition.
Sequelae were recorded for 7 patients: Two developed hearing impairment or loss and 5 suffered visual defect, headache, paresis, hydrocephalus and unspecified sequelae. Thus, a total of 32 did not develop any sequelae.
Notification
Any physician receiving an MD patient for treatment shall immediately notify the case when clinical suspicion arises. Notification is by phone to the Danish Health and Medicines Authority's Medical Officers of Health in the region where the patient is admitted and in writing using Form 1515 to Statens Serum Institut, Department of Infectious Disease Epidemiology.
Prophylaxis for contacts
Household-like contacts to patients with suspected or verified MD are offered antibiotics prophylactically, EPI-NEWS 17/10.
Vaccination is offered via the Medical Officer of Health following verified MD caused by group A, B, C, W135 or Y to the group of persons who have been offered antibiotics prophylaxis EPI-NEWS 33/14. Vaccination must be given no later than 4 days after exposure.
Two tetra-valent conjugate vaccines have been registered for protection against meningococcal disease caused by group A, C, Y or W135; Nimenrix® and Menveo®.
Nimenrix® can be used for children aged ≥ 1 year of age and for adults. The vaccine is administered as a single dose.
Menveo® can be used for children aged ≥ 2 year of age and for adults. The vaccine is administered as a single dose.
If indicated, children aged 2 months to 1 year may receive primary vaccination in the form of 2 Menveo® doses given at a minimum 1-month interval. The Danish Medicines Agency (now the Danish Health and Medicines Authority) has previously assessed that the vaccine may be used off-label in this age group, EPI-NEWS 37/10. In case of continued risk of exposure, a booster dose is given 12 months after the primary vaccination programme. Vaccination of children aged from 2 months to 1 year should be limited to cases in which administration of the four-valent vaccine (Menveo®) is indicated.
Bexsero® is approved for protection against MD of group B in persons above 2 months of age.
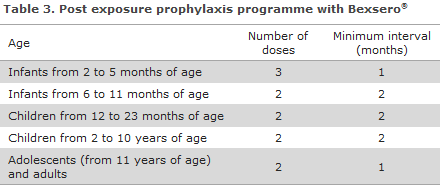
If the child continues to be at risk, a booster should be given, cf. EPI-NEWS 33/14.
The vaccine requires an issue permit from the Danish Health and Medicines Authority, which you can apply for electronically at The Danish Health and Medicines Authority's website.
Commentary
The 44 notified cases of MD was the lowest number recorded since monitoring of the disease began in 1980. As in previous years, the MD incidence was highest in the younger-than-1-year age group. Figure 1 presents the decreasing incidence of group B from 1993 to 2012, and then a limited increase which is why group B is once again the most frequently occurring type (59%).
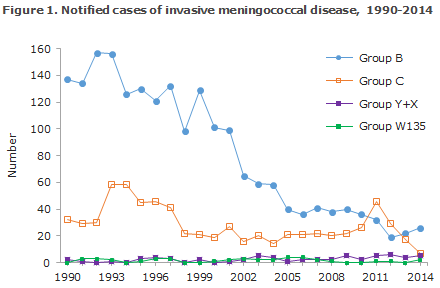
The number of cases caused by group C continued to decrease (from 46 cases in 2011 to 7 in 2014). Group C comprised 14% of MD and is therefore at par with group Y (11%), which - following a limited increase in 2011 - has remained stable.
W135 is a rare group, as only 1 or no annual cases have been observed since 2007. 2014 saw 2 cases belonging to this group; and another 2 cases of W135 have been recorded in 2015 (the most recent case in late July). An increase has been observed in the incidence of W135 in the UK from 22 cases in 2009 to 117 cases in 2014. This increase is due to spreading of a specific clone called: men W:c11.
This clone was also seen in connection with recent outbreaks in Brazil, Argentina and Chile, among others. In response to the increase observed in the UK, a vaccination programme with tetravalent conjugate vaccine for the 13-18-year–olds was introduced as this age group has the highest incidence of the carrier state and therefore also has an increased MD risk. In Denmark, the incidence of W135 remains low.
Changes have been introduced in the manner in which the annual report is prepared, relating to risk factors and sequelae related to MD. Previously, it was standard practice that the notifying physician noted on Form 1515 if the patient had any risk factors for development of meningitis and if the patient subsequently developed any sequelae.
As this workflow was resource-consuming - not least for the notifying physicians - and as it did not always produce the desired degree of detail, a pilot was performed to compare the information achieved from Form 1515 with the information which can be achieved through data extraction from selected diagnose codes from the National Patient Register (NPR) for patients with pneumococcal meningitis in 2012.
The project demonstrated that the extraction from the NPR provided the needed information, and therefore the information in the 2014 annual report for both purulent meningitis and meningococcal disease was collected in this automatic manner and supplemented with information from the notification form, and this procedure will be followed in the future.
(S.S. Voss, C.H. Suppli, P. Valentiner-Branth, Department of Infectious Disease Epidemiology; S. Hoffmann, Department of Microbiology and Infection Control)
Purulent meningitis 2014
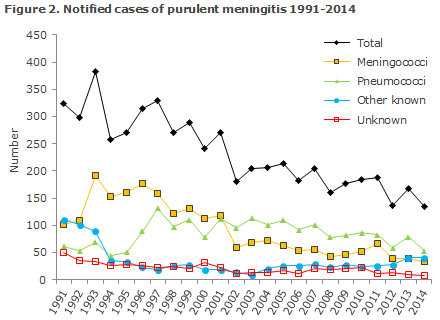
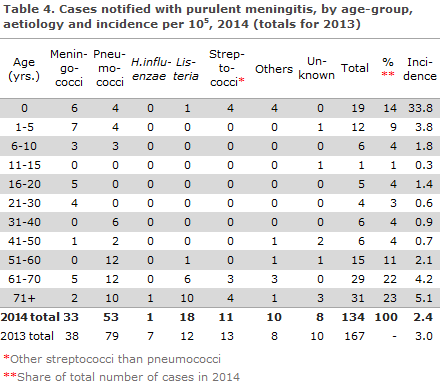
2014 saw a total of 134 notified cases of purulent meningitis. Figure 2 and Table 4 show the distribution by aetiology and age. Meningococcal meningitis (33 cases) in 2014 is described elsewhere in this issue of EPI-NEWS.
Pneumococci
2014 saw a total of 53 notified cases of meningitis with Streptococcus pneumoniae. This figure included 35 cases detected by culture of cerebrospinal fluid (CSV), 10 by PCR testing of CSV (in 6 of the cases, pneumococci were also detected by blood culture), 7 cases by a combination of clinical meningitis and finding of pneumococci through blood culture, and in 1 case by clinical meningitis and finding of pneumococcal antigens in the CSV.
For 19 patients, information was available on underlying conditions and/or dispositions: Three had cancer, 3 were immunocompromised, 6 had an infection (including 3 with pneumonia and 2 with acute otitis media, and 1 had had pneumococcal meningitis 5 months previously (of a different type)), 1 was premature, 1 had suffered a traumatic lesion to the brain many years earlier and 5 had chronic illness.
Nine patients died due to the condition and 13 suffered late sequelae: One patient suffered a serious disease course with hydrocephalus and insertion of a ventriculo-peritoneal shunt, 2 had visual defects, 5 had hearing impairment or loss, 3 had epilepsy, 1 learning disabilities and 1 presented with unspecified nervous system symptoms. A total of 31 persons experienced no sequelae due to the condition.
Other streptococci
Meningitis due to other streptococci than pneumococci was detected in 11 cases.
These cases included 5 cases caused by haemolytic group B streptococci (GBS) and 6 cases with detection of non-haemolytic streptococci (4 cases of S. mitis, 1 case of S. salivarius and 1 case of S. anginosus).
Of the 5 cases of GBS, 4 occurred in neonates. Three of these were probably infected perinatally, and the last neonate may have either been infected through breastfeeding or perinatally as GBS was detected in the mother's breast milk and in her urine (detected by culture). None of the affected children were premature. Two suffered a complicated disease course, but no sequelae were subsequently recorded for any of the children. The fifth case was a 70+-year-old immunocompromised patient with heart disease who suffered sequelae in the form of epilepsy and renal impairment.
Among the cases with non-haemolytic streptococci, 5 of the 6 patients had underlying disease and/or risk factors; 1 had cancer, 1 heart disease, 1 had an endocrine condition and kidney disease, 1 had previously had intracranial pathological tissue resected, and 1 had endocarditis and liquorrhea. All patients were > 60 years of age. One died due to the condition, the remaining 5 recovered with no sequelae.
Haemophilus influenza
A single case of H. influenzae was reported (a non-capsular strain, which therefore could not be grouped; Biotype 1) in a patient above 70 years of age who, according to the notification, had a dural defect and who had also had meningitis repeatedly. The patient suffered sequelae in the form of hearing loss.
Listeria monocytogenes
A total of 18 cases caused by L. monocytogenes were notified. Seventeen cases had become infected in Denmark, and the country of infection for the final case was unknown. One case of congenital listeria meningitis was seen in a premature child, and another case was observed in a 52-year-old patient - the remaining cases occurred in patients ≥ 65 years of age. Underlying disease and/or risk factors were registered for 14 of the patients (cancer, heart/lung disease or kidney disease). Eight patients died due to the condition, 2 developed sequelae and 8 did not have any sequelae.
Other aetiology
Two adults had meningitis where Staphylococcus aureus was detected. None of the two had underlying disease, but one had a pre-existing pneumonia. Both recovered without sequelae.
A 64-year-old patient with cancer developed meningitis with Staphylococcus epidermidis following resection of intracranial pathological tissue. No sequelae have been recorded.
Seven patients had meningitis caused by Escherichia coli, including 4 neonates (2 of whom were premature). It is presumed that these patients were infected through the birth canal. Three adult patients all had underlying disease and/or risk factors; 1 had a shunt due to benign increase in intracranial pressure, 1 had previously had intracranial pathological tissue resected and 1 had heart and kidney disease. Three died due to the condition (1 infant and 2 adults). Another infant developed sequelae in the form of impaired vision and hydrocephalus, whereas the other 3 recovered with no sequelae.
Unknown aetiology
In a total of 8 cases, patients were notified on the basis of clinical tests and/or cell counts and microscopy of cerebrospinal fluid consistent with purulent meningitis, but without detection of bacteria by culture or PCR.
Underlying disease and/or risk factors were recorded for three of the patients; 1 with heart disease, 1 with arthritis and 1 with both cancer, abuse issues, pneumonia, heart and lung disease and arthritis. No patients developed sequelae.
Commentary
2014 saw a total of 134 notified cases of purulent meningitis, which is the lowest number of cases recorded since 1991.
The number of notified cases of pneumococcal meningitis has followed a decreasing trend from the approx. 100 annual cases observed in the 2000-2007 period before the introduction of the seven-valent conjugate pneumococcal vaccine (PCV7) into the childhood vaccination programme EPI-NEWS 37a+b/07 to 53 cases in 2014. Furthermore, an approx. 30% decrease was observed in the incidence of invasive pneumococcal disease in the entire population; from 1,056 annual cases in the 2000-2007 period to 734 cases in 2014. None of the 8 cases of pneumococcal meningitis in children aged 0-5 years of age were caused by the serotypes that form part of the 13-valent conjugate pneumococcal vaccine, which has formed part of the childhood vaccination programme since 2010.
Meningitis caused by other streptococci than pneumococci decreased from 13 cases in 2013 to 11 cases in 2014. The number of cases of purulent meningitis of other known aetiology has increased by 5 cases since 2013. This is due to an increase in the number of listeria meningitis cases caused by a major listeria outbreak in 2014, EPI-NEWS 1-2/15. The share of cases with an unknown aetiology continued to decrease, by 8 cases in 2014 compared with 10 cases in 2013.
The annual report for 2014 was prepared in a different manner than previously. Changes were introduced relating to the validation of cases and to the collection of knowledge about the patients' risk factors and sequelae following meningitis. The change relating to the collection of knowledge about risk factors and sequelae is described in the above 2014 annual report on meningococcal disease.
(S.S. Voss, C.H. Suppli, P. Valentiner-Branth, Department of Infectious Disease Epidemiology; T. Dalby, Department of Microbiology andInfection Control)
New guidelines from the Danish Health and Medicines Authority on the handling of HUS and VTEC
VTEC, verocytotoxin-producing Escherichia coli (E. coli) are diarrhoea-producing E.coli bacteria that can form verocytotoxin. The clinical picture associated with infection covers a range of symptoms from transient, non-bloody diarrhoea to severe, bloody diarrhoea with intense abdominal pain, and serious complications like kidney failure (haemolytic uraemic syndrome (HUS)) and death. VTEC may be categorised according to O group/serotype, toxin type and other virulence factors. In Denmark, the most frequently occurring O groups are O26, O103, O117, O145, O146 and O157 – together they constitute 64% of the verocytotoxin-producing E. coli. The probability that an infection is complicated by HUS is primarily associated with the virulence profile, and Statens Serum Institut regularly performs risk assessments of specific VTEC strains' association to HIS. An updated list of HUS-associated VTEC is available at the SSI website.
The Danish Health and Medicines Authority has amended the guidelines on handling of patients with HUS and VTEC infections. The new guidelines propose a differentiated handling of these patients. Previously, all VTEC bacteria were handled in the same manner, but the new guidelines recommend that patients who are infected with VTEC types not associated with a risk of developing HUS be handled in line with the guidelines that normally apply to infectious diarrhoea. This means that in these low-risk VTEC infections, a control culture will normally not be recommended after the diarrhoea has ended, and that the patient may attend work or day-care, etc., once symptom-free.
For high-risk VTEC, i.e. E. coli bacteria, for which the infection may be complicated by HUS, the tightened guidelines remain in place. In these cases it is essential that patients who have tested positive to these VTEC subtypes are not received in day-care institutions or work in sensitive occupations before they have tested negative at least 2 times.
The new guidelines replace the previous "Guideline for doctors' notification of illness caused by haemolytic uraemic syndrome (HUS) and verocytotoxin-producing bacteria (VTEC)" (no. 61 of 14/4/2000).
The Danish Health and Medicines Authority recommends that children below 7 years of age who present with diarrhoea and patients with bloody diarrhoea, regardless of their age, see a doctor and become tested for VTEC. As antibiotic treatment of patients with VTEC infection may increase the risk of developing HUS, antibiotics should not be used. Furthermore, antidiarrhoetics should not be used.
(F. Scheutz, Department of Microbiology and Infection Control), L. Müller, K. Mølbak, Department of Infectious Disease Epidemiology)
Link to previous issues of EPI-NEWS
16 September 2015