No 44 - 2014
Legionella pneumonia 2013
New hepatitis A vaccine from the SSI
Legionella pneumonia 2013
Notified cases
There were a total of 114 notified cases of legionella pneumonia (LP) with symptom onset in 2013; among these, 73 cases were laboratory-verified in accordance with the criteria described in EPI-NEWS 40/09.
A total of 74 (65%) were men; the median age was 66 years for men and 68 years for women. A total of 82 cases were presumably infected in Denmark, as patients for whom the country of infection was not stated are counted as if they had been infected in Denmark.
The median age for these was 68 years (range 38 to 92 years), and 50 (61%) were men. For 27 patients, a minimum of one predisposing factor was reported, two had no known predisposing factors, and in 53 cases information on predisposition was missing or the risk was unknown. Sixteen patients died.
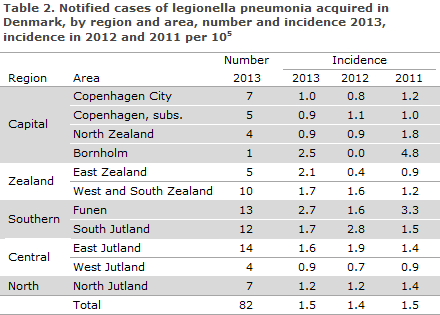
Infection category is presented in Table 1, whereas the number and incidence per 100,000 for the notified cases of legionella pneumonia acquired in Denmark in 2013 by region and area and compared with the incidences for 2012 and 2011 are presented in Table 2.
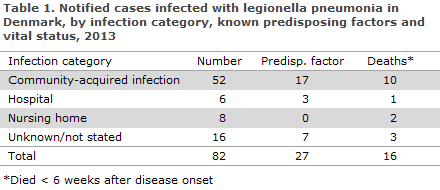
Community-acquired infection
In a total of 52 cases with presumed community-acquired infection in Denmark, 19 cases were culture-verified. In eight cases, it was known that a suspected water supply in the person's home, workplace or another suspected source of infection had been tested. Only in one case did the typing result from the patient's isolate and water samples from the patient's home match, which allowed the source of infection to be confirmed. A total of four persons are believed to have been infected through contact to water at their place of work, two of whom were culture-positive.
Institutionally acquired infection
A total of 14 cases of presumed or certain infection via institutions were notified, six from hospitals and eight from nursing homes. Three of these 14 patients died in connection with their legionella infection. Seven were culture-verified. We have knowledge of water sample testing in only one of the culture-verified cases, and the result did not indicate that the institution's water was the source of the infection.
Infection acquired abroad
A total of 32 cases were probably infected during travels abroad, two of whom died. A total of 25 of the cases were notified with the ECDC. For more information on cases infected abroad in 2013, please see EPI-NEWS 26/14.
Laboratory-confirmed cases
Legionnaires' disease can be diagnosed by culture, legionella antigen urine test (LUT), titre increase in serum immunoglobulin or by PCR. The SSI has knowledge of a total of 113 laboratory-confirmed cases of LP in 2013, including 40 cases that were confirmed by PCR only, of which two only tested positive to Legionella ssp. (non-pneumophila). All cases were notified. Legionella spp. was isolated by culture from 41 patients. The distribution of legionella isolates was as follows: 25 L. pneumophila sg 1 (17 Pontiac and 8 non-Pontiac), nine sg 3, one sg 5, three sg 6, one sg 10 and in one case, the sg could not be determined. L. bozemanae was isolated from one patient.
Possible outbreaks
No new outbreaks were recorded in 2013. However, since 2010 the Kolding area has seen an increased LP incidence compared with the preceding ten years and compared with other Danish cities, EPI-NEWS 41/13. Primarily, L. pneumophila sg 3 and sg 1 infections (with the unique DNA type ST 931) have been observed. As previously, 2013 saw an increased incidence (8.7 per 105) as a total of five cases residing in the 6000 zip code area were notified. Among these cases, four were detected by PCR, whereas the fifth case was confirmed by culture and found to be an sg 1 DNA type ST 931 case.
Commentary
2013 saw the lowest number of notified cases of legionella pneumonia since 2005, and the lowest number of culture-verified cases since 2001. Cases are handled differently depending on whether they were acquired abroad or in Denmark where we distinguish between community-acquired and nosocomial infection. Overall, the distribution on these infection categories and on L. pneumophila serogroups was in line with those observed in previous years. However, it is an increasing trend that cases need to be classified as infection category not stated; in 2013 this group comprised 14% (16) of all cases.
This is due to the fact that the SSI frequently receives notifications (sometimes incomplete) so late that it is no longer relevant to perform epidemiological follow-up to establish the infection category. A third of the cases observed in 2013 were diagnosed by PCR only. PCR is not considered a confirmatory analysis, why only the remaining two thirds of the cases were confirmed as legionnaires' disease (through LUT or culture). Culture should always be attempted on PCR-positive specimens.
(C. Kjelsø, Department of Infectious Disease Epidemiology, S. Uldum, Microbiology and Infection Control)
New hepatitis A vaccine from the SSI
Following supply issues, Statens Serum Institut replaces its provider of hepatitis A vaccines. As from Week 45, the SSI will therefore provide the hepatitis A vaccine Vaqta® manufactured by Sanofi Pasteur MSD. Prices and pack sizes are available at the SSI's website.
Vaqta® is an inactivated vaccine in which the active antigen content is measured as international units (IU), and vaccines contain either 25 or 50 IU per dose.
Vaqta® is indicated for protection against hepatitis A in children over 1 year of age and in adults. Children aged from 1 year to 17 years of age shall be given the paediatric dose (corresponding to 25 IU), and adults from 18 years and above shall receive 1 ml (corresponding to 50 IU). The vaccine is administered intramuscularly to the deltoid region. In patients with reduced clottability, the vaccine may be administered subcutaneously in the upper arm.
To ensure long-term protection, a booster dose is to be given. The booster should be given 6-18 months after the initial dose, if possible, but even if the interval between the first and the second dose of hepatitis A vaccine exceeds 18 months, there is no need to reinitiate the vaccination. The booster dose is decided on the basis of the person's current age rather than the age when the first vaccine was given. If, for example, a person receives the first Vaqta pæd® at 17 years of age and then returns for the booster after turning 18 years old, the adult dose should be given.
Vaqta® can be used interchangeably with other inactivated hepatitis A vaccines as the first and second (booster) dose. Corresponding data are not available for Vaqta pæd®.
The vaccine is contraindicated in persons with known hypersensitivity to one or more of the ingredients. This vaccine may contain trace amounts of neomycin and formaldehyde as these are used in the production process.
Vaqta® should be administered with caution in breast-feeding women. For information on vaccination of pregnant women, please see EPI-NEWS 26a/13.
The most frequently reported adverse reactions are mild and short-lasting. They include fatigue, pain at the injection site and fever. For further information, please see the summary of product characteristics.
Based on a mathematical model and extrapolation from antibody decay, the protective effect will endure for a minimum of 25 years in a minimum of 99% of all fully vaccinated persons.
(L.K. Knudsen, P.H. Andersen, Department of Infectious Disease Epidemiology)
Link to previous issues of EPI-NEWS
29 October 2014