No 40/41b - 2024
Screening of pregnant women for hepatitis B, HIV, and syphilis, 2023
Screening of pregnant women for hepatitis B, HIV, and syphilis, 2023
- In 2023, 94 pregnant women were found to have hepatitis B, 20 had HIV, and 10 had confirmed syphilis during pregnancy screening.
- In 2023, 15 of the 20 pregnant women with HIV were previously diagnosed with HIV and were in treatment.
- Seven out of the 10 pregnant women with syphilis were Danish.
- All pregnant women, including those with known hepatitis B infection, should be screened for all three diseases, as a positive test result from pregnancy screening requires action in the delivery room.
- All pregnant women with hepatitis B, syphilis, or HIV should be referred to a specialized department.
- Midwives and other healthcare personnel who vaccinate newborns of hepatitis B carrier mothers against hepatitis B must register this in FMK (Shared Medication Record) / DDV (The Danish Vaccination Register).
- The general practitioner must complete the vaccination of children born to hepatitis B carrier mothers and ensure serological examination of the vaccination effect in the children one month after the end of the vaccination series.
Screening of pregnant women for hepatitis B virus (HBV) was introduced on November 1, 2005, and for HIV and syphilis on January 1, 2010. An annual report for 2023 is now available.
In 2023, 62,705 blood type analyses were performed among pregnant women. Almost all of these (99.9%) were tested for hepatitis B virus, HIV, and syphilis. The majority of pregnant women choose to accept the offer to be screened for hepatitis B, HIV, and syphilis, thereby enabling the prevention of almost all potential cases of mother-to-child transmission of the three diseases. This makes Denmark a unique country and is fully in line with WHO’s goal of triple elimination of congenital syphilis, HIV, and hepatitis B. This is particularly relevant today, as the number of cases of congenital syphilis is rising in countries like the USA.
All detected cases of HIV, hepatitis B, and syphilis must be reported via the SEI2 system.
All women found with hepatitis B in pregnancy screening in 2023 were born abroad or are second-generation immigrants born in Denmark before the introduction of general pregnancy screening. In 2023, 70% of pregnant women found with syphilis were Danish. This shows that syphilis in Denmark is not exclusively a disease among men who have sex with men or immigrants from high-prevalence countries but has re-emerged among heterosexual Danes.
In 2023, four women never underwent an extended, confirmatory syphilis test despite several attempts to trace the patients.
Upon a positive screening test result, the pregnant woman should be informed that most positive screening tests are false positives, but an extended analysis is required to confirm or refute the result.
The pregnant woman should also be informed that if she has syphilis, HIV, or hepatitis B, she can be treated to prevent the child from being infected. Additionally, the pregnant woman should be informed about the importance of her sexual partner(s) also being tested and, if necessary, treated.
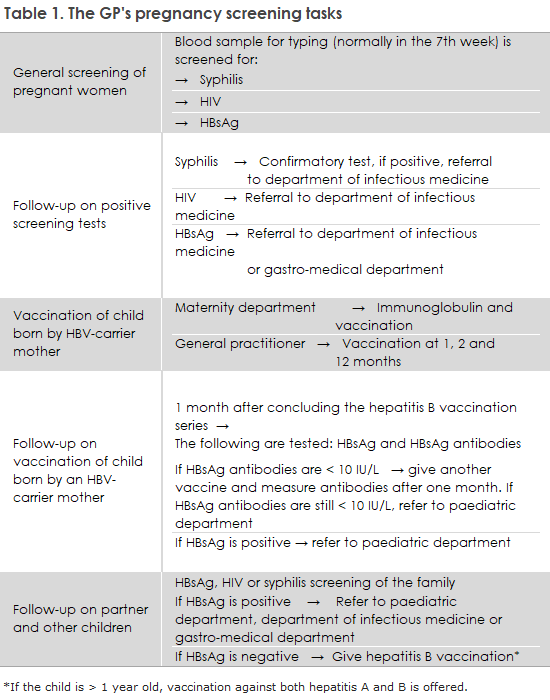
The general practitioner’s tasks in connection with pregnancy screening
The general practitioner’s tasks in connection with the screening are shown in Table 1.
All pregnant women should be offered screening for HIV, hepatitis B, and syphilis
Even if it is known to the pregnant woman and her general practitioner that she has HIV or chronic hepatitis B, it is important that the pregnant woman is offered full pregnancy screening. This is because the communication between Statens Serum Institut (SSI) and the maternity wards, which need to be advised to administer and later document the administration of hepatitis B vaccine and immunoglobulin to newborns of carrier mothers, relies on the information SSI receives from the laboratories performing the pregnancy screening blood tests.
Pregnant women who are undocumented immigrants can be seen by a doctor at one of the Red Cross health clinics in Copenhagen and Aarhus. Pregnancy screening is also offered here, but this is not covered by the public maternity care offer. Maternity wards should be aware of taking blood samples for testing for syphilis and hepatitis B, as well as a rapid HIV test, if a woman with unknown status is admitted. See the Health Authority’s guidelines on general screening of pregnant women for infection with hepatitis B virus, human immunodeficiency virus (HIV), and syphilis.
Pregnant women with positive test results should be referred to a specialized department
It is important that general practitioners refer pregnant patients who have been diagnosed with one of the three infections to a specialized department, even if the patient does not have symptoms of infection, as shown in Table 1. Just as the pregnant woman should be treated for HIV and syphilis, pregnant women with high HBV viral load (>106 IU/ml) can often be offered treatment around week 28, which can reduce the risk of intrauterine transmission, according to guidelines for the treatment of hepatitis developed by the Danish Society for Infectious Diseases and the Danish Society for Gastroenterology and Hepatology.
The pregnant woman’s partner and other children should be called in for a blood test according to the Health Authority’s guidelines on general screening of pregnant women for infection with hepatitis B virus, HIV, and syphilis.
According to the Health Authority’s 2013 guidelines on HIV and hepatitis B and C viruses, all children born to HBV carrier mothers who are vaccinated against hepatitis B at birth and later by their general practitioner should be tested for both vaccination effect and current infection one month after the end of the vaccination series, as shown in Table 1, i.e., when the child is 13-15 months old.
This is necessary because some children are infected in utero, and in these cases, the vaccine has no effect. It is the responsibility of the general practitioner to arrange this examination, as shown in Table 1. If the child is infected, they should be referred to the pediatric department. If the child is inadequately covered (anti-HBs <10 IU/L) despite the vaccinations, another vaccine can be given, after which antibodies are measured one month later. If there is still insufficient protection, the child should be referred to a pediatric department. Statens Serum Institut sends reminder letters to general practitioners about hepatitis B vaccination of children born to hepatitis B carrier mothers and subsequent testing of the children, EPI-NEWS 25/2024.
Hepatitis B vaccination must be registered at birth in FMK/DDV
It is mandatory for doctors, nurses, and midwives who administer the vaccine to register this in FMK/DDV.
Previously, it was a problem that newborns were not found in FMK/DDV until a few days after birth. This has been changed so that the children can now be looked up by the assigned CPR number immediately after birth.
Hepatitis B vaccination and the vaccination course termed “Hepatitis B post-exposure for neonates” (In Danish: ”Hepatitis B post-eksposition til nyfødte”) are added under Add new vaccination (In Danish: “Opret ny vaccination”) in the FMK/DDV (vaccination registry). Immunoglobulin cannot be registered in the FMK/DDV as it is not a vaccine.
When a vaccination course is created, both the child’s parents and the general practitioner will automatically be able to see the dates for when the next vaccination should be given.
Reminder letters about vaccination of children born to women with chronic hepatitis B
Reminder letters about vaccination of children born to women with chronic hepatitis B have been sent via e-Boks to the children’s mothers and their general practitioners since June 2024. The new dispatch procedure will ensure that both parties continue to be reminded of the important vaccination and contribute to the digitization process that will make the work easier. Read more about the new dispatch procedure and the intervals for when reminder letters are sent in EPI-NEWS 25/2024.
(L.H. Holm, S. Cowan, M. Wessman, Department of Infectious Disease Epidemiology and Prevention)