No 18/19 - 2022
Two new pneumococcal vaccines approved for persons aged 18 years and above
Purulent meningitis 2021
Two new pneumococcal vaccines approved for persons aged 18 years and above
Two new conjugated pneumococcal vaccines have been approved by the European Medicines Agency and have been awarded a marketing authorisation in Denmark. The two vaccines protect against 15 (Vaxneuvance, MSD) and 20 different pneumococcal serotypes (Apexxnar, Pfizer).
New provisions on subsidies for persons aged 18 years and above who carry an especially high risk of invasive pneumococcal disease
Persons who carry an especially high risk of invasive pneumococcal disease (IPD) are offered vaccination free of charge with a 23-valent polysaccharide pneumococcal vaccine (PPV23) and are additionally recommended vaccination with a conjugated pneumococcal vaccine. On 2 May 2022, new provisions on subsidies were introduced as a limited subsidy is now given for Vaxneuvance (PCV15) for persons aged 18 year and above who carry an especially high risk of IPD, whereas the limited subsidy for Prevenar 13 (PCV13) has been discontinued for this group. A limited subsidy is still in place for PCV13 in persons below 18 years of age who carry an especially high risk. For more information about the new subsidy provisions, please see the website of the Danish Health Authority.
Enhanced duty of notification
As the vaccines have not previously been used, they are subject to stricter reporting requirements. This means that physicians have an obligation to report all presumed side effects in persons who have received vaccination. The stricter reporting requirements will remain in place for two years as from the marketing authorisation was granted.
Presumed side effects are to be notified to the Danish Medicines Agency no later than 15 days after the doctor has become aware of the possible side effect, and they can be reported through www.meldenbivirkning.dk.
Other healthcare workers as well as vaccinees and their relatives may also report any presumed side effects to the Danish Medicines Agency. When notifying, it is important to specify the vaccine’s batch number, if possible.
Vaxneuvance
Vaxneuvance protects against 15 pneumococcal serotypes and is approved for persons aged 18 years and above. The vaccine is approved for prevention of pneumonia, meningitis and sepsis caused by pneumococcal bacteria, and a limited subsidy is given to persons who carry an especially high risk of invasive pneumococcal disease who are 18 years or older. In the period from 2016 to 2021, the 15 serotypes have caused approx. 26% of all IPD cases in persons aged 65 years and above.
In several studies, the effect of Vaxneuvance was compared with the effect of Prevenar 13 by measuring increases in antibody titers following vaccination. For persons aged 50 and above who have not previously received pneumococcal vaccination, an increase was observed in antibody titers that was comparable for the 13 serotypes that are included in both vaccines. For the two serotypes that are unique to Vaxneuvance (22F, 33F), the effect was larger after vaccination with Vaxneuvance, which shows that vaccination with Vaxneuvance provides a broader protection. Similar effects have been found in other age groups and in risk groups, but no studies have compared the clinical effect of the two vaccines. Please see the summary of product characteristics for more information.
Vaxneuvance is administered intramuscularly as a single dose, and the need for booster vaccination has not been established. Citizens who have previously received Prevenar 13 are not recommended vaccination with Vaxneuvance.
The most frequent side effects following vaccination with Vaxneuvance are redness, swelling, itching and soreness at the injection site, headache, muscle and joint soreness, fatigue and fever.
Vaxneuvance cannot be ordered with the SSI as the limited subsidy is given to vaccines dispensed by prescription. A prescription specifying the limited subsidy can be redeemed at pharmacies. For more information about the vaccine, please see the summary of product characteristics.
Apexxnar
Apexxnar protects against 20 pneumococcal serotypes and is approved for persons aged 18 years and above. The vaccine is approved for prevention of conditions caused by pneumococcal bacteria, including pneumonia, sepsis and meningitis. In the period from 2016 to 2021, the 20 serotypes have caused approx. 62% of all IPD cases in persons aged 65 years and above.
The effect of Apexxnar has been studied in several trials comparing the effect to that of Prevenar 13 for the 13 serotypes shared by the two vaccines and with Pneumovax for the 7 serotypes shared between those two vaccines. As is the case for Vaxneuvance, the effect of Apexxnar was studied through antibody measurements. In one of the trials, participants aged 60 years who had not previously received pneumococcal vaccination were randomised to either Apexxnar followed by placebo or Prevenar 13 followed by Pneumovax at a one-month interval. The immune response caused by the 13 serotypes that are found in Prevenar 13 and in Apexxnar was comparable for the two vaccines. For the remaining seven serotypes that are included in both Pneumovax and Prevenar 13, the immune response was comparable for six of the serotypes, whereas the effect on serotype 8 was lower for Apexxnar than for Pneumovax. The clinical significance of this remains unknown. Please see the summary of product characteristics for more information.
Apexxnar is administered intramuscularly as a single dose, and the need for booster vaccination has not been established.
The most frequent side effects following vaccination with Apexxnar are redness, swelling, soreness at the injection site, headache, fatigue, muscle and joint pain and fever.
Apexxnar can be ordered with the SSI in coming weeks, and we expect to stock the vaccine as from Week 22. For more information about the vaccine, please see the summary of product characteristics.
(F. Kristensen Lomholt, Department of Infectious Disease Epidemiology and Prevention)
Purulent meningitis 2021
For a detailed description of the incidence, please see the 2021 annual report on purulent meningitis.
- 2021 saw a total of 86 notified cases of purulent meningitis. This was a slight increase compared with 2020, when a record-low number of cases was notified (73).
- The increase in disease occurrence was mainly due to an increased occurrence of meningitis caused by Haemophilus influenzae, which increased from four cases in 2020 to a record-high 19 cases in 2021. Even so, the trend for the occurrence of the vaccine-preventable serotype b (Hib) was stable with three cases in 2021 compared with two cases in each of the two preceding years.
- The number of meningitis cases caused by pneumococci remained at a stable and low level in 2021 with 45 cases, compared with 41 cases in 2020.
- In 2021, 15 of 45 cases (33%) of pneumococcal meningitis were caused by serotypes included in the 23-valent pneumococcal vaccine (PPSV23). Three of the 15 persons had been vaccinated with the PPSV23. The offer of free vaccination with the 23-valent pneumococcal vaccine (PPSV23) for all persons aged 65 years or above who belong to defined risk groups was extended in 2021 and remains in place.
- The number of cases with purulent meningitis caused by Listeria monocytogenes continued to follow a decreasing trend in 2021 as three cases were recorded. 2019 recorded ten cases and 2020 seven cases.
- In 2021, one case of vaccine failure was notified among persons with purulent meningitis. The case was a two-year-old child who developed meningitis caused by Haemophilus influenzae serotype b (Hib) despite having received three vaccinations in accordance with the childhood vaccination programme.
- In 70% of the purulent meningitis cases, other underlying diseases or risk factors were recorded for the patient.
- In 2021, the overall mortality for purulent meningitis was 10% (nine deaths). The corresponding figures for 2019 and 2020 were 17% (18 deaths) and 14% (ten deaths), respectively.
Meningococcal meningitis in 2021 (six cases) will be described in detail in a future issue of EPI-NEWS and in the 2021 annual report on meningococcal disease.
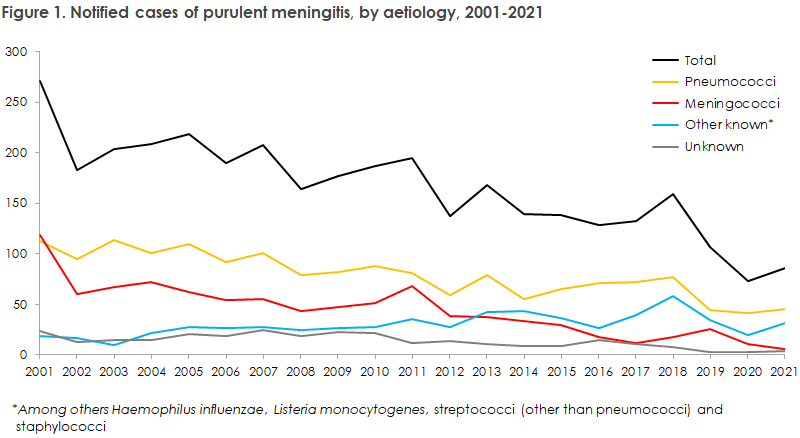
As presented in Figure 1, 2021 witnessed a slight increase in the number of purulent meningitis cases (86 cases) compared with the record-low level observed in 2020 (73 cases). Even so, the number was lower than the level observed prior to 2020. The years 2020 and 2021 were characterised by a number of comprehensive and prolonged social restrictions in connection with the corona epidemic, which presumably contributed to this decline.
Only 55 of the 86 cases were notified. This is a much lower share than in previous years. The low number of notifications is probably an oversight due to the high workload caused by the corona epidemic.
The increase in the number of purulent meningitis cases was mainly due to an increased number of Haemophilus influenzae cases, increasing from four cases in 2020 to 19 cases in 2021. This is the highest number of Haemophilus influenzae cases recorded since data started being recorded in 1994. Even so, the trend for the occurrence of the vaccine-preventable serotype B (Hib) was stable with three cases in 2021 compared with two cases in each of the two preceding years. Vaccination against Hib became part of the childhood vaccination programme in 1993.
At the SSI website Figures and Graphs” (Danish language: Tal og Grafer), it is possible to follow a wide range of diseases and also the vaccination coverage in the childhood vaccination programme and the influenza vaccination scheme.
In 2021, 15 of 45 cases of pneumococcal meningitis were caused by serotypes included in the PPSV23. Three of the 15 patients had been vaccinated with the PPSV23; all three were 60-69 years old and had been vaccinated in 2020. Persons who have turned 65 years old and persons who are at a specially high risk of invasive pneumococcal disease are offered pneumococcal vaccination free of charge (PPSV23). At the SSI website, you can read more about the vaccination offer for persons who are at a specially high risk of invasive pneumococcal disease.
The number of cases with purulent meningitis caused by Listeria monocytogenes continued to follow a decreasing trend in 2021 as three cases were recorded. 2019 recorded ten cases and 2020 seven cases. The figures are small, but a general decline was observed in food-borne conditions during the corona epidemic. Presumably, this is owed to a combination of the changed travel patterns of Danes, fewer medical consultations and the general corona restrictions, i.e. closing of restaurants and canteens.
The number of deaths related to purulent meningitis is defined as the number of deaths within 30 days after the time of diagnosis. In 2021, a total of nine deaths related to purulent meningitis were recorded, corresponding to a 10% mortality. Thus, mortality was lower than in 2019 (17%) and 2020 (14%).
In 2021, a digital solution was launched for notification of purulent meningitis and other notifiable diseases. This is described in more detail in EPI-NEWS 38/39 - 2021. Find information about how to notify an infectious disease electronically in the guide “How to notify infectious diseases” at the SSI website (in Danish language).
(N.U. Friis, P. Valentiner-Branth, Department of Infectious Disease Epidemiology and Prevention, S. Hoffmann, H-C. Slotved, K. Fuursted, Department of Bacteria, Parasites & Fungi)