No 37 - 2018
CPO guideline and mandatory notification
Information about influenza vaccines for the 2018/19 influenza season
Ordering 3-valent and 4-valent vaccines
CPO guideline and mandatory notification
Carbapenemase-producing organisms, CPO - new guideline on handling and executive order on notification published on 5 September 2018
In recent years, Denmark has seen an increase in the occurrence of infections with carbapenemase-producing organisms (CPO), EPI-NEWS 44/17. Outbreaks have been reported both in and between hospitals, which is a worrying development.
On this basis, the Danish Health Authority has made CPO notifiable cf. Executive Order on the notification of CPO Detected in Persons (in Danish language). Simultaneously, the authority has published a guideline on the prevention of CPO spreading. The guideline was prepared in collaboration with the relevant medical societies and Statens Serum Institut.
For more information, please see the website of the Danish Health Authority where the executive order, guideline and appendices are available (in Danish language). Tables describing risk situations and investigation and isolation are provided as separate files, and a short version of the guideline is available.
Background
CPO are multi-resistant bacteria that are resistant to the antibiotics normally used in the treatment of infections. CPO comprises two main groups: 1) intestinal bacteria: carbapenemase-producing enterobacteria (CPE), e.g., Escherichia coli and Klebsiella pneumoniae, and 2) environmental bacteria: Pseudomonas aeruginosa and Acinetobacter baumannii. The term CPE is used to designate intestinal bacteria. Thus, CPE are a subset of CPO.
The three main principles in the prevention of CPO and other multi-resistant bacteria are:
- diagnostics
- strict adherence to infection control guidelines
- reduction of antibiotics consumption.
Testing of persons who are at an increased risk of having CPO is essential, and positive samples must be submitted to Statens Serum Institut for characterisation and monitoring.
Main tasks to prevent CPO from spreading
The guideline focuses on the healthcare sector’s efforts to limit the spreading of CPO in hospitals, at nursing homes and other places of accommodation for sick and debilitated persons. This is so because persons who are already sick or debilitated have a greater risk of becoming infected and of running a serious disease course.
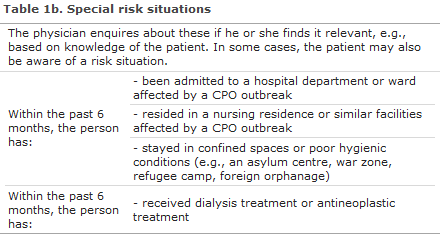
Anyone admitted to hospital must be asked if they are in one of the general risk situations, see Table 1a. If the patient confirms this, he or she is tested for CPO. If it is known that one of the special risk situations is relevant (Table 1b), the patient also undergoes CPO testing.
For planned admissions, the patient is tested for CPO at the pre-admission visit to the outpatient clinic, or at the GP. Samples are submitted no less than one week before admission to ensure that the results are available on the day of admission. Negative test results must be less than 4 weeks old.
CPO testing includes rectal swabbing or a stool sample. If relevant, samples are taken from ulcers, entry sites of foreign objects, stoma, urine (if a urinary catheter was used), tracheal secretion (if the patient was intubated) and localisations where CPO was previously observed.
For same-day surgery and outpatient testing and treatment at hospitals and clinics, the patient should not be asked about CPO risk situations.

Treatment of carrier state
Currently, we have no documented methods to eliminate a CPO carrier state. As the sensitivity of CPO sampling is low, a negative test result does not provide certainty that the patient is not a carrier. Therefore, strict adherence to the general infectious hygiene guidelines is essential in preventing spreading.
The Danish Health Authority has prepared an information letter that may be handed out to CPO carriers.
Infection control guidelines
The guideline is based on strict adherence to standard precautions that include correct hand hygiene, use of a work suit and personal protection equipment, re-processing of instruments/devices, cleaning of rooms and inventory, handling of patient excretions and handling of dirty laundry and waste. At the website of Statens Serum Institut, separate appendices are available for hospitals, nursing residences and similar institutions and for home care and home nursing care services.
At hospitals, CPO-positive patients receive care in a private room (isolation), and they should make use of a separate toilet.
Persons who receive primary-sector treatment, nursing home dwellers and citizens who receive home-nursing care are not isolated. Persons who are not admitted to hospital may participate as normally in social activities and rehabilitation, etc. To the extent possible, the citizen must be instructed in correct hand hygiene and, where relevant, receive assistance to perform correct hand hygiene. If visitors take part in the care, it is recommended that they use personal protection equipment (disposable gloves and a disposable lab coat). Healthcare workers who perform treatment and nursing tasks in the patient’s residence are covered by the same infection control guidelines on hand hygiene and personal protection equipment as healthcare workers at hospitals.
Mandatory notification
To facilitate monitoring of developments and implementation of measures, notification is now mandatory.
Any doctor who tests a person for CPO must notify in writing all cases where CPE has been detected (intestinal bacteria that are carbapenemase-producing), regardless of whether the case is a clinical infection or asymptomatic carrier state. Notification is done using the Danish Health Authority’s Form 1515. Notification must be made to Statens Serum Institut and to the unit within the Danish Patient Safety Authority that handles tasks where the patient is currently staying.
Please note that the Danish Health Authority’s Form 1515 has been updated to facilitate CPO notification.
Laboratories performing CPO tests for persons must regularly notify any detection of all types of CPO to Statens Serum Institut and must submit the isolate to Statens Serum Institut. For more detailed guidance, please see the website of Statens Serum Institut.
The guideline and appendices are available at www.sst.dk and www.ssi.dk
(A. Kjerulf, B. Kristensen, L. Porsbo and M.B. Ilan, Central Infectious Hygiene Unit, A.M. Hammerum, H. Hasman and U.W. Sönksen, Reference Section for Antibiotic Resistance, all from Statens Serum Institut B. Søborg, T. Rønne, S.U. Jacobsen, Danish Health Authority)
Information about influenza vaccines for the 2018/19 influenza season
This year it has been possible to acquire a limited number of doses of a 4-valent influenza vaccine. Therefore, a 4-valent as well as a 3-valent vaccine will be available for the influenza season. The 3-valent vaccine protects against the three types of influenza virus - two influenza A types and one influenza B type - that the WHO assesses are the most likely types of influenza virus to be circulating in the coming influenza season. The 4-valent vaccine protects against an extra influenza B type.
The 4-valent vaccine may be offered to the two following risk groups:
- Pregnant women in their second or third trimester (until the end of February 2019)
- Persons below 60 years of age who, upon medical assessment, have
- chronic disease
- severe obesity
- other serious conditions
See Executive Order on Free Influenza Vaccination (in Danish language)
The distribution is based on knowledge that influenza B virus usually - and in contrast to the influenza A virus types - more frequently gives rise to disease in school children and young adults.
Furthermore, young people generally respond better to influenza vaccination than elderly people do.
In addition to the mentioned risk groups, it will be possible to give approx. 10% of the available 4-valent vaccines to a limited group of especially vulnerable elderly people aged 60 years of age or more. For the latter group, the individual physician’s medical assessment will decide how the 4-valent vaccines that are available to each medical practice or vaccination clinic will be distributed.
(A.K. Devantier and B. Søborg, Danish Health Authority)
Ordering 3-valent and 4-valent vaccines
The 3-valent (650,000 doses) and 4-valent influenza vaccines (100,000 doses) should be distributed in a manner that ensures that all physicians/vaccinators can vaccinate the risk groups listed by the Danish Health Authority.
Therefore, an upper limit has been defined for how much 4-valent influenza vaccine you can order. The limitations are based on how large a share of the target group GPs and vaccination clinics have vaccinated in the previous influenza season.
The following distribution principle has been suggested:
General practitioners: Up to 20% of the total order placed may comprise 4-valent vaccines. If, e.g., a total of 250 doses of influenza vaccines are ordered, a maximum of 50 doses of 4-valent influenza vaccine may be ordered, and 20 packs of 3-valent vaccine (200 doses).
Vaccination clinics, including vaccinating pharmacies: Up to 10% of the total order placed may comprise 4-valent vaccines. If, e.g., a total of 1,000 doses of influenza vaccines are ordered, a maximum of 100 doses of 4-valent influenza vaccine may be ordered, and 90 packs of 3-valent vaccine (900 doses).
Hospital pharmacies: May contact the SSI Order Office and order a limited amount of 4-valent influenza vaccine to cover current needs.
Generally, we encourage our clients to check their patient lists for those who will be receiving the vaccines to ensure that their orders reflect the specific number of people expected to need 3-valent and 4-valent vaccine, respectively.
Nevertheless, it will be possible to reorder 4-valent vaccines for pregnant women in their second or third trimester until 1 March 2019.
The SSI will be making changes to a limited number of orders for 4-valent influenza vaccines
The SSI has revised the orders made so far/the order patterns, and has learned that a number of clients are not adhering to the published guidelines. The SSI therefore expects to make changes to some of the received orders.
The clients will not be informed before changes are made. Any changes made will be stated on the delivery note that is handed over along with the goods ordered.
The SSI will be making changes according to the following guidelines:
No changes will be made to orders that are in line with recommendations.
If 4-valent vaccines have been ordered only, the SSI will check how many influenza vaccines the client acquired in the previous season and possibly adjust the share of 4-valent vaccines accordingly.
The remaining clients will have their orders adjusted based on an overall assessment of the number of 4-valent vaccines ordered and the amount of 4-valent vaccines that are available for those orders when standard recommendations have been followed.
Additional information on influenza vaccination will be published in EPI-NEWS no. 39/18.
(The SSI Order Office, Statens Serum Institut)