No 42-43 - 2017
Novel 9-valent HPV vaccine in the childhood vaccination programme
Hepatitis A vaccines in short supply
Paperless notification of syphilis and gonorrhoea is now possible
Novel 9-valent HPV vaccine in the childhood vaccination programme
|
As previously mentioned, EPI-NEWS 39/17, all girls who initiate HPV vaccination under the childhood vaccination programme as from 1 November 2017 are vaccinated using Gardasil®9. The Gardasil®9 vaccine prevents cervical cancer and precursors to cervical cancer and HPV-related anal cancer, cancer around the opening of the vagina and precursors to these, as well as genital warts. A total of 90% of all cervical cancer cases are caused by the HPV types 16, 18, 31, 33, 45, 52 or 58, and 90% of all condyloma cases are caused by HPV type 6 or 11.
The vaccination programme
Girls who are under 15 years at their first vaccination receive two doses. The minimum interval separating the two doses is 5 months, and the vaccination series should be completed within 13 months. If these intervals are not observed, a total of three doses are to be given.
In this case, the minimum interval between the second and third dose is 3 months.
From 15 years upwards, three 0.5 ml doses are administered at 0, 2 and 6 months. The minimum interval separating the 1st and 2nd vaccination is 1 month, and the 2nd and 3rd vaccinations should be given at a minimum interval of 3 months. If possible, all three vaccinations should be administered within a year, and if one vaccination is delayed, the next should be given as soon as possible. The vaccination series should never be re-initiated.
Girls who are immunosuppressed at the time of vaccination are recommended a 3-dose programme.
Programme for young women who have not concluded their HPV vaccination
Since no studies have been conducted to explore the safety, antibody level or vaccine efficacy of a combination of the three HPV vaccines, we recommend concluding the vaccination schedule with the vaccine with which you have initiated vaccination.
Young women who initiated their vaccination series receiving Cervarix® should complete their entire series with Cervarix®.
If there continues to be young women who started their vaccination series with Gardasil®, they should conclude vaccination with Gardasil®9 at the intervals recommended for Gardasil®9, Table 1. Whether or not a person may conclude vaccination in a 2- or 3-dose programme depends on the age at the first vaccination, the minimum and maximum intervals between the vaccinations, and which vaccine was administered.
- If one dose of Gardasil® was given at the age of 9-13 years, and a maximum of 12 months have passed since the initial vaccination, one dose of Gardasil®9 is given.
- If the first dose of Gardasil® was not given before the age of 14 years, a 3-dose-programme is given.
-If only one dose of Gardasil® was given, and the current age is below 15 years, two doses of Gardasil®9 are given at a 5-13-month interval.
- If only one dose of Gardasil® was given, and the current age is 15 years or above, two Gardasil®9 vaccines are given at a 3-month interval.
- If two doses of Gardasil® were given and the young woman has not concluded her vaccination, i.e. if she started her vaccination course at 14 years of age or the two vaccinations were given at an interval of less than 6 months or more than 12 months, one Gardasil®9 vaccine is given at a minimum interval of 3 months, as described in Table 1.
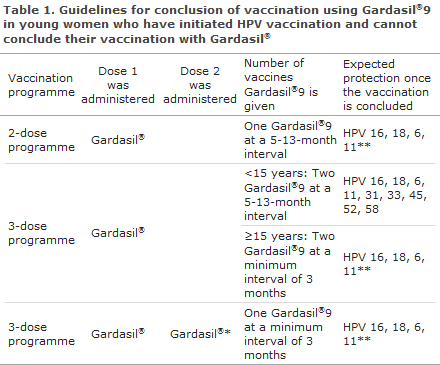
*Vaccination not concluded even though two doses were administered, see the Figure for a description of how the vaccines are given, including specific intervals.
**Long-term protection may only be expected against the HPV types that form part of the HPV vaccination with which you have initiated the vaccination series.
Presumably, a number of young women have started their vaccination series with Gardasil®, without concluding the series, and have then initiated vaccination with Cervarix®. We recommend that these young women conclude their vaccination programme with Cervarix®.
Service codes
Specific administrative service codes have been created:
1. Gardasil®9 = 8328
2. Gardasil®9 = 8329
3. Gardasil®9 = 8330
As usual, to complete vaccination with Cervarix ®, the following is used:
2. Cervarix® = 8335
3. Cervarix® = 8336
The service codes are used for settlement with the region, but do not free the physician of the duty to notify the vaccination to the Danish Vaccination Register.
The HPV vaccine and injection
The vaccine contains virus-like protein particles (VLP) from HPV types 6, 11, 16, 18, 31, 33, 45, 52 and 58. The virus-like particles are produced in yeast cells using recombinant DNA technology and are adsorbed on amorphous aluminum hydroxyphosphate-sulfate adjuvant (0.5 milligram AI).
The vaccine should be shaken vigorously before use. At delivery, the vaccine may appear as a clear liquid with a white precipitation. After thorough agitation, the vaccine becomes a cloudy, white liquid.
The vaccine is given intramuscularly. The preferred site is the deltoid area of the upper arm or the higher anterolateral area of the thigh. Concurrent administration of other vaccines has been tested for a combination booster vaccine with diphtheria and tetanus given with either pertussis and/or polio. HPV vaccine may also be given concurrently with other vaccines, but these vaccines must be given at separate injection sites.
Further information about the HPV vaccine is available at "vaccineleksikon" (in Danish language).
Adverse effects
The adverse effects that were reported in connection with the clinical trials with Gardasil®9 are the same as those that were observed in connection with the two other HPV vaccines, Cervarix® and Gardasil®.
Reactions at the injection site and influenza-like symptoms like headache, fever, fatigue, pain, vertigo and nausea are the most frequently occurring adverse effects. The reactions are generally mild and transient.
In the clinical trials, more cases were reported of local reactions at the injection site with redness and swelling for Gardasil®9 than for Gardasil®. The number of reported serious side effects was identical for the two vaccines.
Like several of the other vaccines in the childhood vaccination programme, Gardasil®9 contains an aluminum adjuvant. An adjuvant is a substance that makes the vaccine more effective but also increases the risk of local reaction at the injection site in the form of, e.g., soreness, swelling and redness. Gardasil®9 contains more aluminum than Gardasil®, but the same amount as Cervarix® (0.5 mg Al).
Contraindications
The vaccination is contraindicated in patients with allergies to the active substances or the excipients.
HPV vaccination must be postponed in patients with acute conditions causing a high fever. An ordinary cold with a low fever is not a contraindication and should not postpone vaccination.
Studies assessing the effect of Gardasil®9
In the eight clinical trials that form the basis of the authorisation of Gardasil®9, the vaccine was compared with the previous Gardasil® vaccine, for which solid studies have already demonstrated a preventive effect against HPV infection and cancer precursors.
These comparative studies have shown that the Gardasil®9 vaccine is as effective as Gardasil® with respect to the four HPV types 6, 11, 16 and 18, and that it also prevents infection with the HPV types 31, 33, 45, 52 and 58.
In one of the trials including 14,204 women between 16 and 26 years of age, it was studied how many participants developed cell changes related to the five additional HPV types 31, 33, 45, 52 and 58. The trial focused on the 12,033 women who had not already become infected with the five HPV types prior to their first vaccination. A total of 6,016 of women received Gardasil®9, while 6,017 women received Gardasil®. After 5 ½ years, one woman in the Gardasil®9 group had developed cancer precursors caused by one of the five additional HPV types. In the group of women who were given Gardasil®, 38 developed cancer precursors.
Post-vaccination protection
In order to determine how long the vaccine remains effective, those who were vaccinated with Gardasil®9 in the clinical trials will be followed for 10 years. It will be studied if the HPV antibodies persist and if cell changes develop.
Preliminary results after 3 years of follow-up show that the vast majority is still protected against the nine HPV types, but the effect is expected to last considerably longer than 3 years. The full duration of the protective effect and any need for further vaccination at a later stage are unknown.
The HPV vaccine protects only against conditions caused by HPV types 6, 11, 16, 18, 31, 33, 45, 52 and 58. Relevant preventive measures against other sexually transmitted diseases must therefore also be used after vaccination.
The HPV vaccine is not indicated for treatment of cancer, dysplastic lesions or venereal warts.
HPV vaccination does not replace routine screening for cervical cancer, which remains very important.
Enhanced duty of notification
On 1 November 2017 when Gardasil®9 is introduced into the Danish childhood vaccination programme, it will - as any other new medicine - be covered by an enhanced duty of notification of any presumed adverse effects. This enhanced notification duty will remain in place for a 2-year period as from the marketing was initiated. Therefore, all presumed adverse effects of the vaccine must be reported by physicians to the Danish Medicines Agency. Gardasil®9 was granted its marketing authorisation in June 2015, but was not marketed in Denmark until March 2017. Once the 2 years have passed, i.e. as from March 2019, doctors will have an obligation to notify any serious or unknown adverse effects that were presumably caused by Gardasil®9.
The Danish Medicines Agency also encourages other healthcare workers as well as the vacinees and their relatives to report any presumed adverse effects to the Danish Medicines Agency. When notifying, it is important to specify which HPV vaccine was given and to provide the vaccine’s batch number, if possible.
Adverse effects may be reported to the Danish Medicines Agency at: www.meldenbivirkning.dk
Ordering Gardasil®9
Gardasil®9 is delivered as 10x1 doses and may be ordered using item number 99204 on Form 6 or at ordre@ssi.dk. A maximum of 2 packages may be ordered per provider number in the first order.
Registration in the Danish Vaccination Register
All vaccinations, including Gardasil®9, shall be registered in the Danish Vaccination Register (DVR) in pursuance of Executive Order on Access to and Registration etc. of Medicinal Product and Vaccination Information.
Commentary
Since the beginning of 2017, more than 24 million doses of Gardasil®9 have been distributed worldwide. Hereof, 9 million doses were distributed in the US and the remaining 15 million doses in the rest of the world. Gardasil®9 was authorised by the US health authorities in December 2014, and the vaccine was authorised by the European medicines authorities in June 2015. Gardasil®9 forms part of or will form part of the publicly paid vaccination programmes in Austria, Canada, Croatia, Italy (12 of 20 regions), the United States, Germany, New Zealand, Macau, Portugal, Slovenia, Australia (as from January 2018) and the Czech Republic (as from January 2018). The tender that brought Gardasil®9 to Denmark will be valid for a 4-year-period.
(P. Valentiner-Branth, P.H. Andersen, Department of Infectious Epidemiology and Prevention, B. Søborg, the Danish Health Authority)
Hepatitis A vaccines in short supply
Several European countries are currently experiencing a shortage mainly of monovaccines for protection against hepatitis A, for children as well as for adults.
The shortage has arisen, among others, after one of the main manufacturers needed to discard a substantial number of hepatitis A vaccine batches two years ago. Increased consumption due to ongoing outbreaks of the disease in Europe in men who have sex with men (MSM), among others, may also have contributed.
The UK health authorities and the European Centre for Disease Prevention and Control (ECDC) have prepared detailed guidelines on how to ensure sufficient protection against hepatitis under these circumstances.
Statens Serum Institut (SSI) is responsible for ensuring the supply of vaccines in Denmark. To ensure that, e.g., people who are specifically exposed to the infection may be vaccinated, part of the total stock is placed in so-called blocked storage, which is activated only if no more vaccines are available from the freely available storage.
As described on the SSI website, several hepatitis A-containing vaccines are currently on back order. We expect that several of these vaccines will be supplied in the course of November but in limited amounts, and therefore a risk exists that new shortage situations may arise in months to come.
How can the available hepatitis A-containing vaccines be used most appropriately?
The ECDC and the WHO concur that persons who have received the first hepatitis A-containing vaccine with sufficient antigen content in connection with travels to areas with an increased risk of hepatitis A can wait for up to 5 years before concluding their vaccination and that such persons may be expected to enjoy protection against hepatitis A also when engaging in further travels to endemic areas within this period. This applies when a pure hepatitis A vaccine (e.g. Vaqta®, Havrix®) is given, and also when an adult dose of the combination vaccine against both hepatitis A and hepatitis B for children (adult dose Twinrix®/Ambirix®) is given, see Table 2.

Children aged as from 1 year up to and including 15 years may be vaccinated with either the paediatric dose of the combination vaccine against hepatitis A and B (Twinrix paediatric®, 3-dose programme) or the adult dose of the combination vaccine against hepatitis A and B (Ambirix®, 2-dose programme).
After the first dose of Twinrix paediatric®, the majority of children (89%) will be protected against hepatitis A after one month, but in practice they will enjoy protection from the day they initiate their travel owing to the long incubation period of the condition; and as there is currently no shortage of Twinrix paediatric®, we recommend that vaccination be concluded using this vaccine.
After the first dose of Ambirix®, the child is expected to be protected against hepatitis A for up to 5 years. It may be considered to possibly postpone the rest of the vaccination course until the supply of hepatitis A vaccine has stabilised.
Children who have received a full vaccination series against hepatitis B (Infanrix Hexa® or Hexyon/Hexaxim®) under the childhood vaccination programme have already received 3 doses of hepatitis B-containing vaccine. Data support that up to 5 doses of hepatitis B containing vaccine may be given to children without increasing their risk of adverse effects. When wanting to protect a child against hepatitis A because he or she is at an increased risk of transmission, the child may be vaccinated with the 2-dose programme (Ambirix®) to achieve full protection against hepatitis A.
Children of carrier mothers who are vaccinated against hepatitis B at birth have received 4-5 hepatitis B-containing vaccines. Although no data support that these children may safely be given another 2-3 hepatitis B-containing vaccines, there is no reason to presume that further vaccination with Twinrix® or Ambirix® may increase the risk of adverse effects. This applies to children of carrier mothers, other children and to adults alike.
Adults (> 15 years) may be vaccinated with a combination vaccine against hepatitis A and B (Twinrix®, 3-dose programme). After the first dose of Twinrix®, nearly all vacinees (94%) will be protected against hepatitis A after one month, but in practice they will be protected from the day they initiate their travel owing to the long incubation period of the condition.
As an alternative to protection against hepatitis A via vaccination, you may, as a last resort, use human immunoglobulin which, nevertheless, provides only short-time protection, and which will frequently be a more expensive alternative than vaccination, given the updated dosage recommendation of 0.17 ml/kg.
(A.H. Christiansen, P.H. Andersen, Department of Infectious Disease Epidemiology and Prevention)
Paperless notification of syphilis and gonorrhoea is now possible
Syphilis and gonorrhoea can now be notified directly on the web, with no need for filling in a paper form.
Notification Form 1510 for syphilis and gonorrhea can be filled in directly online, be enclosed to an e-mail and sent to sygdomsanmeldelse@ssi.dk.
It is important that the form is sent to the SSI from a safe digital mailbox (encrypted e-mail) to safeguard any sensitive personal information.
Every GP has a secure digital mailbox associated with his or her authorisation number, and every hospital has a secure digital mailbox for each hospital department. Secure e-mail may be sent via these two options. Additionally, if you want to use a computer that is not already connected to a safe digital mailbox, you can install an extension from NemID and then configure your mail programme to send and receive safe e-mail and download a “key” that is associated specifically with the SSI’s disease notification mailbox: sygdomsanmeldelse@ssi.dk.
For doctors who prefer the previous mailing process, it will still be possible to print out the completed form and then send it by surface mail, or scan it and send it as an attachment to the safe digital mailbox. Pre-paid reply envelopes can also be obtained from sygdomsanmeldelse@ssi.dk or by telephoning section secretary: Linda Roth, +45 3268 3744.
(Department of Infectious Disease Epidemiology and Prevention)
25 October 2017