No 41 - 2017
Invasive pneumococcal disease and coverage of pneumococcal vaccination in the childhood vaccination programme 2016
Report on the HPV vaccination coverage - status as per 4 September 2017
Invasive pneumococcal disease and coverage of pneumococcal vaccination in the childhood vaccination programme 2016
The 7-valent pneumococcal conjugate vaccine (PCV-7) was introduced into the Danish childhood vaccination programme on 1 October 2007, and as from April 2010 it was subsequently gradually replaced by the 13-valent conjugate pneumococcal vaccine (PCV-13) which has superior coverage. A total of three vaccinations are recommended at 3, 5 and 12 months of age.
Laboratory monitoring data preceding the introduction of PCV-7 demonstrated that 64% of all cases of invasive pneumococcal disease (IPD) in children up to 5 years of age was caused by pneumococcal serotypes included in the PCV-7 (4, 6B, 9V, 14, 18C, 19F and 23F) and that 91% of IPD in the same age group was caused by serotypes included in the PCV-13 (the PCV-7 serotypes plus serotypes 1, 3, 5, 6A, 7F and 19A), EPI-NEWS 37 a+b /07.
Before PCV vaccination is defined as the period 2000-7, the PCV-7 period as 2008-10 and the PCV-13 period as 2011-16.
Vaccination coverage
Coverage was calculated using data from the Danish Vaccination Register obtained on 4 September 2017.
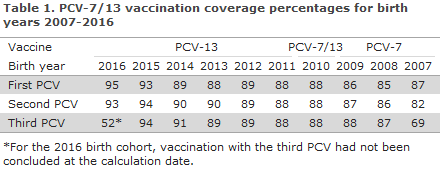
The coverage of the PCV vaccines in the childhood vaccination programme for birth years 2007-2016 was in the range 85-95% for the first PCV, 82-94% for the second PCV, and for 2007-2015 the coverage was 69-94% for the third PCV, Table 1. Modest geographical variation was observed with a slightly lower coverage for the third PCV in the areas of West and South Zealand and in the City of Copenhagen (i.e. in the municipalities of Copenhagen, Frederiksberg, Tårnby and Dragør) compared with the remaining parts of the country, www.ssi.dk/data.
The coverage of the PCV follows an increasing trend and is on a par with the coverage of the DTaP-IPV/Hib / DTaP-IPV/HibHbv vaccination that is administered concomitantly.
Changes in IPD incidence
Figure 1 shows the age-specific incidence of laboratory-confirmed IPD cases per 100,000 inhabitants before and after the introduction of PCV-7 and PCV-13 into the childhood vaccination programme. IPD is primarily seen in very young children and among elderly people.
The report is based on nationwide data from the Neisseria and Streptococcus Reference Laboratory (as from 2000) with the addition of data from the Danish Microbiology Database (the MiBa, as from 2010). An IPD case is defined as a disease episode for which pneumococci (Streptococcus pneumoniae) have been detected in cerebrospinal fluid, blood or another normally sterile sampling material, such as a joint. As from 1 October 2007, submission to the SSI of pneumococcal isolates for serotyping has been required by law to support the national monitoring of IPD.
The total IPD incidence in the population in the period from 2000 to 2007, i.e. before the introduction of PCV vaccination, was approx. 20 cases per 100,000 or an average of approx. 1,060 annual cases. In the PCV-13 period, the incidence has been reduced significantly by approx. 25%, equivalent to 15 cases per 100,000. With 731 detected cases, 2016 has recorded the lowest incidence since PCV was introduced. The decrease in IPD incidence is still observed both among vaccinated and unvaccinated age groups.
The most pronounced decrease was observed in children below 2 years of age whose incidence dropped by approx. 70% in the PCV-13 period (2011-2016) compared with the period prior to the introduction of PCV. This corresponds to a decrease in incidence from 55 cases prior to the introduction of PCV vaccination to 17 cases per 100,000 in the PCV13-period for this age group.
Among children below 2 years of age, the IPD incidence caused by the 6 extra serotypes that are included in the PCV-13 vaccine has decreased from approx. 12-13 cases before PCV vaccination to 0-1 cases per 100,000 in the PCV13-period. Nevertheless, the incidence of IPD caused by pneumococcal serotypes that do not form part of the vaccine has increased significantly from around 5 cases before PCV vaccination to 12 cases per 100,000 in 2016, but this does not overshadow the overall advantages gained owing to the vaccine, Figure 1.
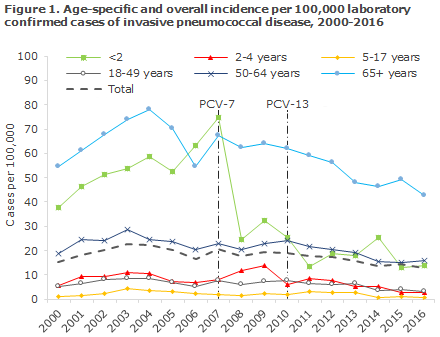
Among persons above 65 years of age, a significant decrease in IPD incidence has been seen; from approx. 66 cases per 100,000 before PCV vaccination to approx. 43 cases per 100,000 in 2016. This decrease is mainly owed to a decrease in the occurrence of the serotypes included in PCV-7 and PCV-13. The observed decrease in incidence among unvaccinated elderly persons is presumably owed to the fact that PCV reduces the carrier state in vaccinated children for the serotypes comprised by PCV and thereby reduces the circulation of vaccine serotypes in the population; the so-called herd effect.
Fluctuations are seen in the IPD incidence recorded for the different age groups. 2015 observed a minor increase for +65-year-olds, whereas the incidence in 2016 was at the lowest level recorded since the introduction of PCV. In 2014, a minor increase was seen for children below 2 years of age, which was due to an increased occurrence of a serotype that is not included in the vaccine. The increase was transitory and short-lived, and the incidences in 2015 and 2016 are on a par with the incidence seen in 2011 and are the lowest incidences seen among children below 2 years of age since the introduction of PCV.
Commentary
The introduction of PCV has had a significant and lasting effect on the IPD incidence in Denmark. The effect of the vaccine is observed both among vaccinated and unvaccinated population groups, but is most pronounced among children below 2 years of age. In addition to the changes in the occurrence of serotypes, which is likely an effect of PCV, the occurrence of pneumococcal disease and the various pneumococcal types vary randomly over time. These changes may, in part, be related to factors that are not owed to vaccination; among others, a varying occurrence of the types not included in the vaccines. Since 2010 and as part of the monitoring efforts, we have included IPD cases recorded in the MiBa only. These are IPD cases that were diagnosed at a department of clinical microbiology (DCM), but where the isolate was not submitted to the SSI for serotyping of where DNA was detected without cultivable isolate. Serotyping is the most valid information available for assessment of the effect of PCV. These lacking cases are limited in number and have not changed the previously reported occurrence of the disease or the overall effect of the vaccine. We would like to take this opportunity to thank all Danish departments of clinical microbiology for their contribution to the national monitoring efforts and for a fruitful collaboration in 2016.
(Z.B. Harboe, T. Dalby, H.-C. Slotved, C.S. Jørgensen and K. Fuursted, Microbiology and Infection Control, L.K. Knudsen, P. Valentiner-Branth, Department of Infectious Disease Epidemiology and Prevention)
Report on the HPV vaccination coverage - status as per 4 September 2017
HPV vaccination has formed part of the childhood vaccination programme since 1 January 2009, and has been offered to girls as from 12 years of age; as of now girls from a total of 10 birth years (1996-2005). Furthermore, young women from a total of 11 birth years (1985-95) have been offered vaccination through catch-up programmes. Apart from the 2003-05 birth years, vaccination has enjoyed a high coverage of at least 75% for HPV1 (EPI-NEWS 47/14) for all the birth years of women who have been offered HPV. Overall, a total of 615,426 women and 17,858 men have received a minimum of one HPV vaccine according to the Danish Vaccination Register.
Since 2013, Denmark has had a special focus on notification of symptoms to the Danish Medicines Agency that are not known adverse effects of the vaccine, e.g. prolonged headache and marked fatigue. The medicine authorities have concluded that no association can be established between these symptoms and the vaccine. The negative debate about HPV has resulted in a drastic drop in the vaccination coverage in Denmark, whereas the remaining Nordic countries enjoy a high and stable coverage.
It now seems that the trend in Denmark is turning. Thus, from March to August this year, approx. 4,200 HPV vaccinations have been administered per month compared with 2,200 vaccinations per month in the same period last year.
Figure 2 presents the number of young women who have received the first HPV vaccine in the HPV programme per month in the period from June 2012 to August 2017. The number of vaccinations administered per month is characterised by strong seasonal variation. For all vaccines given in the childhood vaccination programme, fewer vaccines are given in the summer period and in the period between Christmas and New Year. Therefore, the number of vaccines administered is reported using a method, a 6-month rolling average, which smooths out these seasonal variations while making it easier to spot changes in the vaccination activity over time. A decrease has been observed in the monthly number of young women who initiate HPV vaccination from a maximum of 2,900 in October 2012. The decrease is not constant, and fluctuations are seen in the period until April 2015, when a drastic drop occurred. From 2,300 women initiating HPV vaccination in March 2015, a drop to approx. 1,000 women per month is seen in the period from October 2015 to May 2016. From September 2016, more than 1,200 women initiate HPV vaccination per month and a strong increase is seen until August 2017, which is the last month included in the current report. The level of vaccination of nearly 2,500 women who initiate HPV vaccination in August 2017 corresponds to the level seen in October of 2013.
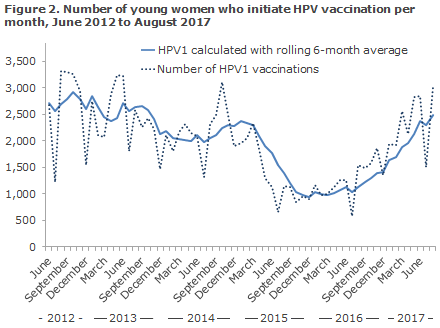
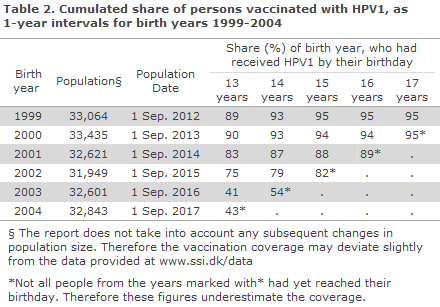
Table 2 shows the share of girls vaccinated with PHV1, by year, for birth years 1999-2005. Nearly 90% of the birth years 1999-2000 had been vaccinated at 13 years of age. For the birth years 2001-2002, who were vaccinated from 2013 onwards, a delay in vaccination is observed. A lower vaccination coverage is also observed at 15 years of age, particularly for birth year 2002. The vaccination delay is also seen for birth years 2003 and 2004, among whom only slightly more than 40% have received the initial HPV at 13 years of age. Even so, for birth year 2003 a strong increase is seen in the share of HPV1-vaccinated persons at 14 years of age, to 54%. This relative increase is higher measured as percentage points than for birth years 1999-2002. It is not possible to assess the relative increase for birth year 2004 as the entire birth year had not turned 14 years old at the calculation date, and the number may therefore be underestimated. The same applies for birth year 2005 as the full birth year had not yet turned 13 years old at the calculation date.
Commentary
Since the end of 2012, a downwards trend has been seen in the number of young women who initiate HPV vaccination. The decrease in the coverage of the HPV vaccination programme peaked after the TV2 documentary “The vaccinated girls”, broadcast in March 2015. From September 2016, a steep increase is seen, and in August 2017 a total of 2,500 women initiated HPV vaccination, which is on a par with the October 2013 level, and close to the highest number of women ever vaccinated in this report, i.e. the 2,900 women who initiated vaccination in October 2012. In August 2017, more than twice as many young women initiated HPV vaccination as in the period from October 2015 to May 2016, when vaccination was at its lowest.
This report also demonstrates a relatively larger increase in the share of girls who initiate their course in the vaccination programme at 14 years of age than at 13 years of age, which is a reflection of a greater share of delayed vaccination. The delay was most pronounced for birth year 2003 and 2004, where less than half were vaccinated at 13 years of age. Even so, for birth year 2003, a strong increase is seen, viz. the share of those who were HPV1 vaccinated at 14 years of age was 54%. This relative increase from 13 to 14 years of age is higher measured as percentage points than for birth years 1999-2002.
HPV vaccination is offered to all young women until they turn 18 years old. The four birth years 2001-2004 have seen a relative decrease in vaccination coverage compared with girls from previous birth years, e.g. 1999-2000. The coverage follows an increasing trend, which gives hope that these, as well as future birth years, may achieve adequate protection against HPV infection. Nevertheless, there is a trend towards more girls initiating their course of the programme at a later age than was the case when the programme was implemented. The Danish Health Authority recommends vaccination at 12 years of age to ensure protection of HPV before onset of sexual activity.
(C. Suppli, P. Valentiner-Branth, Department for Infectious Disease Epidemiology and Prevention, K D Bjerre, Data integration and Analysis, K. Mølbak, Infection Preparedness)
Link to previous issues of EPI-NEWS
11 October 2017