No 49 - 2014
Outbreak of enterovirus D68 among children in the US and Canada and occurrence in Denmark
Outbreak of enterovirus D68 among children in the US and Canada and occurrence in Denmark
Enterovirus (EV) of subtype D68 (EV-D68) has been known since 1962 and is one of more than 100 non-polio-like enteroviruses. In 2002, EV-D68 was re-classified from a rhinovirus 87 to the EV group. Previously, EV-D68 had only been seen as sporadic cases and minor outbreaks counting up to 130 cases across the world. In Denmark, less than 10 cases have been observed over the past 5 years.
EV-D68 can give rise to a broad spectrum of symptoms ranging from trivial airway symptoms such as coughing, catarrhal symptoms and in some cases fever, to symptoms more resembling influenza including high fever, headache and myalgia, and also serious pneumonia causing respiratory complications. Finally, in rare cases EV-D68 is associated with aseptic meningitis and paralysis.
In the course of August 2014, first the US and then Canada were affected by a major EV-D68 outbreak. The outbreak started when two paediatric hospitals in Chicago and Kansas City reported an increased number of admissions to their intensive care units due to severe respiratory symptoms. Concurrently, an increase in the number of positive rhinovirus/enterovirus PCR findings was observed in nasal/throat specimens. The outbreak primarily counted children < 5 years of age with underlying chronic disease (e.g. asthma and cystic fibrosis) and/or immunosuppression.
By the beginning of September, the US Centers for Disease Control and Prevention (CDC) detected EV-D68 in the first 30 specimens from children from the mentioned hospitals. Subsequently, the EV-D68 outbreak spread rapidly, and by 28 November a total of 47 American states were affected by the outbreak, which counted > 1,100 laboratory-confirmed EV-D68 cases. Correspondingly, by mid-November, another > 200 laboratory-confirmed EV-D68 cases had been recorded in Canada.
In the same period, the US and Canada also recorded minor outbreaks among children (approx. 90 children in the US and < 10 children in Canada) with nervous system affection including paralysis of central and peripheral muscles. The median age of the children is 8 years.
The American CDC is now trying to determine if there is a connection between EV-D68 and these outbreaks as all of the children have had infectious disease in the days leading up to their initial neurological symptoms (3-16 days before, median time 7 days), and this virus was found in airways secretions from some of the children. As EV-D68 was prevalent across both the US and Canada in the same period, it is possible that the finding of EV-D68 in the upper airways of several children is no more than a coincidence.
Furthermore, the CDC informs that they are currently investigating 11 deaths for a possible connection to EV-D68 infection, and the Canadian health authorities (Public Health Agency of Canada) state on their website that they have confirmed an EV-D68-related death in a young man with known severe asthma.
EV-D68 in Europe
On 25 November, the European Centre for Disease Prevention and Control (ECDC) published a risk assessment on EV-D68 in Europe. According to a European EV-D68 laboratory network in which the Section for Virology Surveillance and Research (SVSR) from Statens Serum Institut also participates, EV-D68 is in circulation in a minimum of 15 European countries, and the genetic structure of the European and American EV-D68 strains is very alike.
The ECDC assesses that EV-D68 represents a moderate risk for the populations of Europe as no reports have been observed of an increase in the number of airway-related admissions at paediatric departments and intensive care units in any EU countries despite the presumed extensive prevalence of EV-D68. The assessment includes a limited number of sporadic reports of EV-D68-associated severe disease in Europe, including paralyses among a total of four children in Norway, France and England, respectively.
EV-D68 in Denmark
In collaboration with the SSI's Routine Diagnostic PCR Section and the Clinical Microbiology Department, Copenhagen University Hospital (Rigshospitalet), the SVSR obtained 60 airway specimens from symptomatic patients (including 30 patients from across Denmark and another 30 patients from the Copenhagen area) and found eight (13%) cases of EV-D68 among these.
Cases ranged in age from 6 months to 61 years and five (63%) were below 5 years of age.
There was a predominance of girls/women (n = 6). The cases were sampled between 24 September and 4 November and are spread out geographically with one case in Jutland, two in South Zealand and five in the Copenhagen area. Among the eight cases, five have underlying disease; three have asthma, one cystic fibrosis and one is receiving chemotherapy. No Danish EV-D68 patients have experienced neurological symptoms. All cases are believed to have been infected in Denmark, and sequencing of the VP2 region indicates that the cases observed in the Copenhagen area may have been caused by introduction of the same virae, whereas the cases from Jutland and South Zealand are genetically further apart, indicating that they may be the result of separate introductions.
EV-D68 diagnostics
EV-D68 may be diagnosed on the basis of EV- and/or rhinovirus-PCR-positive airway secretions.
As the RNA of EV-D68 is very similar to a rhinovirus, airway secretions that test PCR-positive to rhinovirus may contain EV-D68. An EV PCR-analysis of the airway secretion may confirm/dispel this because an ongoing EV-D68-infection is expected to cause EV PCR-positive airway secretion for up to a week after symptom onset.
Throat swabs may be used in cases when the airway secretion is not available, but a negative throat swab does not exclude EV-D68 infection.
The airway secretion or throat swab may be submitted to diagnostic EV testing at local DCMs or directly to the SSI's virology PCR laboratory if local EV testing of airway secretion cannot be performed. EV PCR-positive throat/airway specimens are sent to the SVSR for further characterisation, including EV-D68-typing.
EV-D68 diagnostics is primarily assessed as being relevant for children/adolescents with severe airway infection and:
- underlying chronic pulmonary disease and/or immunosuppression
- paralysis/neurological symptoms of unknown aetiology.
Relevant diagnosis may contribute to the understanding of any protracted courses in children/adolescents with underlying pulmonary disease and may lower the threshold for admission in case of incipient respiratory depression.
Furthermore, the EV-D68 diagnosis is important in relation to differential diagnostic work up as unnecessary use of antibiotics may be avoided.
EV-D68 virological monitoring
The SVSR, Statens Serum Institut, receives the EV- and/or rhinovirus-positive airway specimens that are submitted with the brown monitoring slip during the current outbreak and characterise these further at no additional cost to the person requesting the test.
Commentary
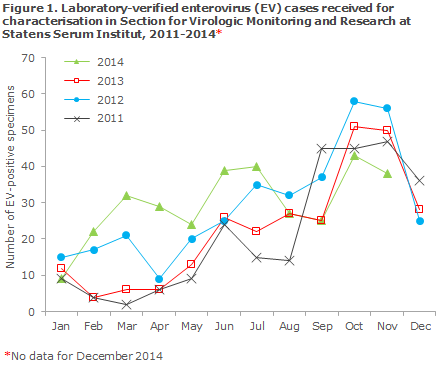
In Denmark, the 2014 EV season has been extraordinary as it has extended throughout most of the year. Even though at present only a limited number of EV-D68 cases have been detected in Denmark, there are probably many more infected persons who have experienced mild symptoms and/or have not been tested for EV-D68. It is currently too early to determine how the outbreak will evolve, but it seems likely that it will subside over the coming weeks as the EV season typically only extends from July/August to November/December, Figure 1.
Speculation as to why EV-D68 is suddenly causing severe airway disease, particularly among vulnerable people, is primarily centred on changes to the virus' antigenicity and the virus' cell-surface-receptor properties. This is so because previously EV-D68 mainly attached to receptors on epithelial tissue in the lower airways, but now it has been shown to have a preference for sialin acid receptors in the upper airways. This goes some of the distance towards explaining why the virus has suddenly become more infectious in humans.
Enteroviruses are monitored at the laboratory level, but also clinically as all new EV PCR-positive patients are asked to provide clinical information on a questionnaire sent out by the Department of Infectious Disease Epidemiology. It is important that the submitted questionnaires are returned to the SSI without undue delay, particularly if the patient has experienced any neurological symptoms and/or paralysis.
(T.K. Fischer, S. E. Midgley, M. W. Poulsen, B. Andersen, Section for Virologic Monitoring and Research, Department of Microbiological Diagnostics and Virology, P.H. Andersen, Department of Infectious Disease Epidemiology, C. B. Christiansen Clinical Microbiology Department, Copenhagen University Hospital (Rigshospitalet)
Link to previous issues of EPI-NEWS
3 December 2014