No 45 - 2013
MiBa – the Danish Microbiology Database
MiBa – the Danish Microbiology Database
MiBa is a nationwide, automatically updated database of microbiological test results. The objectives of MiBa are:
- to provide access for healthcare professionals to microbiological test results from all of Denmark for patients in their care.
- to ensure that the national Danish surveillance of infectious diseases and micro-organisms is performed automatically, flexibly, timely and that it is complete.
- to serve as a shared resource for research projects
- to ensure automatic transfer of data to other databases with a view to monitoring of e.g. antibiotic resistance and hospital infections (HAIBA, www.haiba.dk).
MiBa is the result of collaborative efforts made by the departments of clinical microbiology (DCM), providers of two laboratory ICT systems (MADS and Autonik AB), MedCom and Statens Serum Institut (SSI). MiBa, then, is a collaborative project between the SSI and the DCM. The overall management of the efforts is handled by a Board of Representatives counting the participation of every DCM.
MiBa is financed mainly by a grant from the Danish Ministry of Health from 2009. Since January 2010, all microbiological test reports from the DCMs have been transferred electronically to MiBa. The data transfer does not affect data ownership, and the data therefore remain the property of the involved DCMs/regions.
National display of search results
Only relevant healthcare professionals who are involved in counselling, diagnosing and treating individual patients are allowed to perform MiBa searches. Until the end of 2011, only clinical microbiologists were actually able to access MiBa. Currently, solutions providing access for other relevant groups of healthcare professionals are being implemented.
The objective is for MiBa to become accessible directly from local ICT systems with no extra login requirements. GPs do not have direct access to MiBa, but can find microbiological test results via the Laboratory Portal, which draws on MiBa data. Using an employee certificate, all physicians have access to the Laboratory Portal via Sundhed.dk. GPs can speed up access to the Laboratory Portal via WebReq.
At present, patients do not have access to their own MiBa data, but it is the intention that patients should have access via the Laboratory Portal and Sundhed.dk. For more information on access options to MiBa, please see the MiBa website: www.ssi.dk/MiBa. (in Danish language)
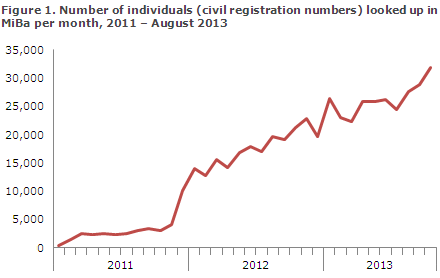
MiBa has now become an integrated part of the daily work routines for a growing number of physicians and nurses, and its use increases in step with the implementation of new access solutions, Figure 1.
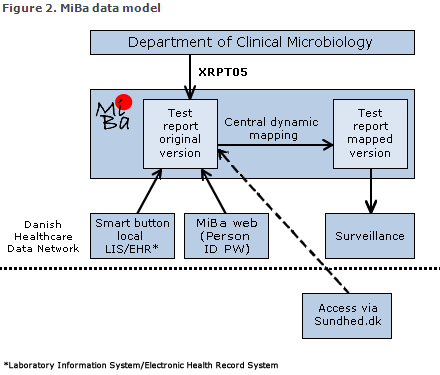
Data model
The principle of MiBa is that whenever a DCM sends a test report to the requestor, an electronic copy is submitted to the database, Figure 2. By means of a standard transfer protocol (MedCom XRPT05) and automatic "translation" from local codes to shared codes (mapping), it is possible to collect data in MiBa with a relatively uniform structure despite considerable local variation.
Surveillance of infectious diseases
Today, strict requirements are in place regarding timely surveillance and control and targeted prevention of infectious diseases. The establishment of MiBa constitutes the initial phase of a modernisation of the national Danish preparedness measures to counter infection.
The SSI's surveillance has so far been based on monitoring of individual clinical cases (through paper forms such as Form 1515) and on notifications from laboratories that had detected micro-organisms or other markers for the conditions in question. This surveillance is based on manual procedures; and notification, submission and reporting of these data therefore generate a considerable workload. Experience has shown that several conditions are under-reported and notifications are frequently late.
Using MiBa for surveillance offers a number of advantages. As data are transferred simultaneously with the clinical test results, data are available for analysis without delay. Any changes in the trends and disease outbreaks may therefore be detected earlier, and any new infectious diseases or changes to known micro-organisms can be monitored as needed.
Detection of a series of micro-organisms is achieved through specific analyses, and for these it is possible to analyse the number of detected cases relative to the number of tests made. Knowledge of the denominator and hence the positive rate will provide improved opportunities to interpret the data and will make it possible to monitor the testing activity in diseases for which this is of particular importance.
The automatic data transfer to MiBa will free physicians and laboratories from the workload associated with data reporting, and this makes it easier to establish voluntary surveillance agreements. Voluntary agreements with the DCMs form the basis of national Danish MiBa-based influenza surveillance; and from this year also Mycoplasma pneumoniae and RS virus surveillance. The figures produced by this surveillance will be reported through INFLUENZA-NEWS.
When comparing data from the previous surveillance scheme with data from MiBa, good agreement has been found for whooping cough and invasive meningococcal and pneumococcal disease, whereas the figures for neuroborreliosis and Clostridium difficile indicate previous under-reporting. The transition to MiBa-based surveillance will be gradual, and the final phase-out of the active laboratory notification and the use of forms will take place some time in the future.
A number of challenges remain with regard to the use of MiBa for surveillance purposes. No national Danish standard exists for the communication of information on subtypes and microbial properties (e.g. toxin production and virulence factors). As these items of information are currently not uniformly structured, standardisation of the data structure is challenging for data originating from various DCMs and from the SSI.
Under the Danish Society for Clinical Microbiology (DCCM), a national collaborative project has been established to solve these issues. Furthermore, other laboratories exist that work with microbiological diagnostics (clinical biochemistry and immunology) and do not report to MiBa. This means that for some virus infections, MiBa data are incomplete. A notification solution including laboratory results from these units is in the pipeline.
Legal framework
Any surveillance will be performed in accordance with Executive Order on Notification of Infectious Diseases (Executive order no. 277 of 14/4/2000) including subsequent amendments. Furthermore, specific resistant micro-organisms are monitored through collaborative efforts made by the DCMs and the SSI (DANRES, a working group under the DCCM).
The Danish Health and Medicines Authority in cooperation with the SSI and other relevant stakeholders are revising the executive order and adapting it to allow for surveillance based extensively on MiBa data.
Applying for MiBa data extraction
MiBa data may be used for research, surveillance and quality assurance projects, but this requires the approval of the DCMs in addition to the official permissions from e.g. the Danish Data Protection Agency. Instructions explaining how to apply for permission to use MiBa data are available on the MiBa website.
Conclusion
MiBa searches provide an overview of microbiological diagnoses made across Denmark and therefore serve to support the diagnostics and treatment of the individual patient. MiBa renders possible far-reaching automatisation of the national Danish surveillance of infectious diseases and will therefore provide a strong starting point for the prevention of infectious diseases.
In future, MiBa will provide unique opportunities for the implementation of national surveillance and research projects.
(M. Voldstedlund, Department of Infectious Disease Epidemiology, the MiBa Board of Representatives and the departments of clinical microbiology).
Link to previous issues of EPI-NEWS
6 November 2013