No 24 - 2013
Malaria 2012
Increasing number of mumps cases in Denmark
Measles outbreak has ended
Novel MMR vaccine in the Childhood Vaccination Programme
THE DANISH HEALTH AND MEDICINES AUTHORITY's guidelines on MRSA and use of antibiotics, in English
Malaria 2012
In 2012, a total of 68 cases of imported malaria were notified by Danish laboratories, Table 1.
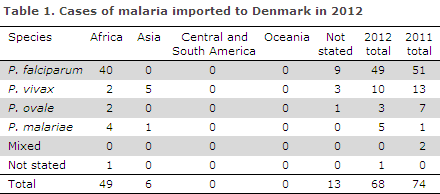
Of the cases for which the presumed country of infection was stated, 89% (49/55) had been acquired during stays in Africa and the remaining (6/55) in Asia. No cases acquired in Central and South America or Oceania were recorded.
Among the cases with a species-specific diagnosis, 73% were caused by Plasmodium falciparum, and these cases were presumably all acquired in Africa. Additionally, the following African cases were reported: two cases of Plasmodium ovale, two cases of Plasmodium vivax and four cases of Plasmodium malariae.
The majority of the cases imported from Asia were caused by vivax malaria, and no cases of falciparum malaria from Asia were reported. The median age was 38 years (range 1 to 71 years). Among the cases for which gender was stated, males comprised 69% and females 31%.
Commentary
The number of notified malaria cases in 2012 was in line with the low numbers seen over the past few years. As previously, the main issue is the risk of the severe falciparum malaria in Africa, particularly among Africans residing in Denmark, who typically spend prolonged periods of time in their countries of origin. Outside of Africa, the risk of falciparum malaria is generally low.
As reported in EPI-NEWS 28-33/12, during the summer of 2012 Greece once again saw local cases of vivax malaria. The risk for Danes travelling to Greece must be considered to be very low, but physicians should remain attentive to and inform travellers to the affected areas of primary malaria prevention: Lakonia, East Attika, Xanthia, Viotia and Karditsa.
The Greek authorities have prepared a national action plan and have intensified monitoring of malaria and other vector-borne diseases with a view to optimizing vector control and diagnosing local malaria cases rapidly and thereby limiting the transmission in the upcoming season as much as possible.
(L.S. Vestergaard, H.V. Nielsen, Reference Laboratory for Malaria, Microbiology and Infection Control)
Increasing number of mumps cases in Denmark
Mumps is caused by infection with parotitis virus. Since the introduction of the MMR vaccine in 1987, the number of mumps (parotitis) cases in Denmark has dropped drastically. In the 2002-2012-period, an average 13 annual cases were notified.
To date, Statens Serum Institut (SSI) has recorded 21 laboratory-confirmed mumps cases in Denmark in 2013. The first case was seen in March when a total of five cases were detected. April, in turn, saw three cases and May a total of 12 cases. At present, only a single case has been observed in June.
The cases are spread across Denmark, but with a clear predominance of cases in the Capital Region of Denmark and Region Zealand, as 12 of the 21 cases were recorded in these regions. Of the 21 laboratory-confirmed cases, notifications have only been received for 14 cases at present.
The age of those who fall ill with mumps has generally increased since the introduction of the MMR vaccine, and the mumps can no longer exclusively be considered a "childhood disease". This also applies to the current cases among whom 14 of 21 are older than 20 years of age.
The risk of complications increases with increasing age. Abroad, major outbreaks have been observed at universities and other educational institutions. Of the 21 cases detected in 2013, three were recorded at military barracks and two at a university. Vaccination status is available for 19 persons, nine of whom had reportedly received one mumps vaccination and three of whom received two vaccinations.
Mumps can be prevented through vaccination with the MMR vaccine which forms part of the Childhood Vaccination Programme. The mumps component of the vaccine is not quite as immunogenic as the two remaining components, but high vaccination coverage will yield good population immunity. Internationally, however, outbreaks have also been described in well-vaccinated population groups.
Physicians are currently encouraged to pay particular attention to the diagnosis in children and younger adults who present with symptoms that are compatible with mumps. Suspicion may be confirmed by a blood sample testing for IgG and IgM antibodies, but the actual diagnosis is established though PCR detection of parotitis virus in a throat swab, nasopharyngeal secretion and/or urine (or blood). Virology Surveillance and Research at the SSI performs typing and sequencing of parotitis virus.
Mumps is individually notifiable on form 1515. The condition is notifiable in cases with a clinical diagnosis and concurrent detection of virus and/or specific antibodies or contact to a known, laboratory-confirmed case.
(L.K. Knudsen, P.H. Andersen, Department of Infectious Disease Epidemiology, T. K. Fischer, Virology Surveillance and Research)
Measles outbreak has ended
The Silkeborg area measles outbreak which started in Week 10, EPI-NEWS 13-14/13, is now considered over. A total of 14 cases were detected. The latest case was unable to transmit infection after 30 April (Week 18), and no further cases have been recorded. Across Denmark, a total of 17 measles cases have so far been detected in 2013. For epidemiological details on the outbreak, please see the SSI web (in Danish language) under "Temaer", “Sygdomsudbrud" and Mæslinger".
(Department of Infectious Disease Epidemiology)
Novel MMR vaccine in the Childhood Vaccination Programme
In pursuance of the provisions on statutory public procurement, the SSI is introducing a new MMR vaccine. The new MMR vaccine, called MMR VaxPro®, is manufactured by Sanofi Pasteur. It replaces the MMR vaccine Priorix® which has been used in the Danish Childhood Vaccination Programme since 2008, EPI-NEWS 38/08.
The vaccines are considered equal and have the same adverse event profile. Children who have been vaccinated once with Priorix® may receive their second vaccination with MMR VaxPro®. As was the case for the previous vaccine, MMR VaxPro® is only available in 10-dose packages and will be handed out as from Week 25.
(B. Neale, Business Development, P.H. Andersen, Department of Infectious Disease Epidemiology)
THE DANISH HEALTH AND MEDICINES AUTHORITY's guidelines on MRSA and use of antibiotics, in English
The following are now available on the English version of the Danish Health and Medicines Authority's web:
The antibiotics guideline, including two information letters, also translated into English:
- Guidelines on prescribing antibiotics
- Guidelines on preventing the transmission of methicillinresistant Staphylococcus aureus (MRSA)
- Treatment of people carrying MRSA
- Information on pig-associated MRSA (398).
(Danish Health and Medicines Authority)
Link to previous issues of EPI-NEWS