No 51b - 2012
Pneumococcal vaccination of persons at increased risk of invasive pneumococcal disease
Pneumococcal vaccination of persons at increased risk of invasive pneumococcal disease
As from 29 October 2012, the Danish Health and Medicines Authority has introduced a limited subsidy (klasuleret tilskud) to cover the 13-valent conjugated pneumococcal vaccine (PCV13). A limited subsidy covering the 23-valent pneumococcal vaccine (PPV23) has been in place for several years. The limitations associated with the two pneumococcal vaccines are identical and include persons with:
- planned/performed splenectomy, organ transplant or cochlear transplant
- splenic dysfunction (e.g. following radiation)
- leakage of cerebrospinal fluid
- previous invasive pneumococcal disease (IPD)
- immunosuppression (e.g. due to HIV infection or lymphoma)
- cyanotic heart disease
- cardiac failure
- palliative surgery for cardiac condition
- chronic pulmonary condition (e.g. cystic fibrosis)
- hypodynamic respiratory insufficiency
- nephrotic syndrome
- immunodeficiencies, excluding agammaglobulinaemia and SCID
In order for the patient to qualify for a subsidy, the physician must mark the prescription “subsidy”, declaring that the person is covered by the limited subsidy.
13-valent conjugate pneumococcal vaccine for persons covered by limited subsidy
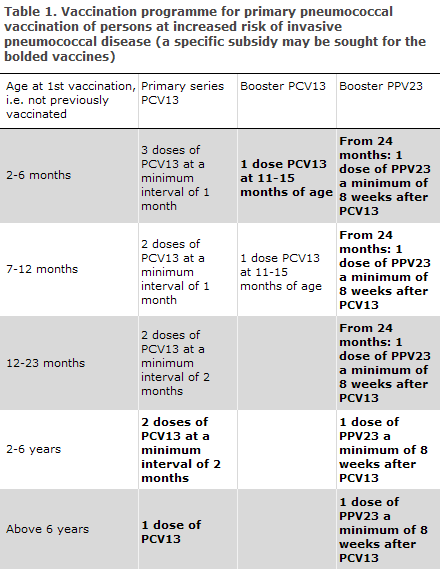
Conjugate pneumococcal vaccination has formed part of the Childhood Vaccination Programme since October 2007, EPI-NEWS 37 a+b/07, and all children born after 30 April 2006 have been offered pneumococcal vaccination. As from week 16, 2010, PCV13 substituted Prevenar (7-valent), EPI-NEWS 15/10. The vaccination programme on pneumococcal vaccination for various age groups covered by the specific subsidy is described in Table 1. In the age groups in which the vaccination programme differs from the standard Childhood Vaccination Programme, it is possible to apply for the specific subsidy for any extra doses. Consequently, it is currently possible to achieve the subsidy for one dose in the 2-6 months age group, two doses in the 2-6 years age group and one dose for children above six years of age and for adults. These doses are bolded in Table 1.
It is recommended that children aged 1-6 years, who were fully immunized with Prevenar (7-valent), receive a single dose of PVC13 to induce immunoreaction against the six additional serotypes. PCV13 triggers a T-cell-mediated immune response and thereby the development of immunological memory. The need for revaccination with a follow-up dose of PCV13 has not been established.
23-valent pneumococcal vaccine for persons covered by limited subsidy
PPV23 is a polysaccharide vaccine covering a total of 23 pneumococci serotypes, including types not covered by the PCV13. PPV23 is expected to prevent approx. 70% of IPD in immunocompetent adults, but no strong documentation exists in support of its preventive capacity against pneumococcal pneumonia.
From the age of 2 to 18 years, it is recommended that a single PPV23 vaccination be given a minimum of eight weeks after PCV13 vaccination is concluded, Table 1. No further vaccination with PPV23 is recommended before reaching the age of 18 years. This is because the serotype coverage in the 5-19 years age group is similar, 61%, for PCV13 and PPV23. Severe local reactions are rare following a primary pneumococcal vaccination with PPV23 and the vaccination therefore needs no previous antibody measurement. This applies even if the person has had repeated pneumococcal infections with resulting production of type-specific antibodies. The pneumococcal vaccination can be given at the same time as influenza vaccination, but at separate injection sites.
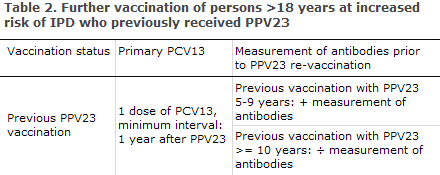
Studies suggest that the vacinee will lose part of the protection achieved over the course of five years, which indicates that revaccination may be needed. However, there is significant individual variation in the duration of protection. Ten years after the primary vaccination, the risk of severe local reactions on revaccination is considered to be small and equal to the risk associated with primary vaccination. In general, revaccination can therefore be conducted ten years after primary vaccination without prior measurement of antibodies, Table 2.
For persons at increased risk, e.g. splenectomised patients, cases of impaired splenic function or patients who are immunodeficient due to other causes, the physician shall assess from case to case if revaccination should be administered sooner. If this is the case, measurement of antibodies should be performed five years after the primary vaccination and prior to any revaccination, Table 2. In patients who are non-responsive to repeated PPV23 vaccination, revaccination will typically not be effective, EPI-NEWS 46/02. Persons with uncertain vaccination status should have antibody levels measured prior to vaccination. Measurement of antibodies is done by submitting a blood sample to the Department of Microbiological Diagnostics and Virology, SSI. Advice on revaccination is issued along with the result of the test.
Sequence of vaccination with 13-valent conjugate pneumococcal vaccine and 23-valent pneumococcal vaccine for persons covered by the limited subsidy
In studies where PCV13 was administered one year after PPV23, the immune response was lower for all serotypes than the immune response following PCV13 given to subjects who had not previously been treated with PPV23. The clinical significance hereof remains unknown, but PCV13 should be given as the first of the two vaccines, EPI-NEWS 2/12. Furthermore, PPV23 should be given a minimum of eight weeks after PCV13 vaccination.
If PPV23 was already administered, a single dose of PCV13 may be given at a minimum interval of one year, Table 2.
Pneumococcal vaccination of adults > 65 years and medical risk groups not covered by the limited subsidy
Since 1997, a number of persons above 65 years have received pneumococcal vaccination in the form of PPV23, EPI-NEWS 46/02. The IPD incidence peaks in this age group. Vaccination is not covered by any programme and patients themselves defray any vaccination costs. Previously, in addition to the age-related indication, several other vaccination recommendations were made concerning persons with a series of medical risk factors, which are not currently comprised by the limited subsidy, e.g. patients who receive treatment or attend follow-up visits for chronic cardiac, pulmonary, hepatic or kidney disease or diabetes mellitus, EPI-NEWS 46/02.
At present, no clinical studies have been made on the effect of a combined pneumococcal vaccination programme aimed at the elderly. Based on what we know about development of immunological memory following PCV13 vaccination, the broader serotype coverage of PPV23 and serological investigations, vaccination of the above-mentioned patient groups should be initiated by PCV13 vaccination. PPV23 should be given a minimum of eight weeks after PCV13 vaccination, Table 1.
If PPV23 was administered previously, a single dose of PCV13 may be given at a minimum interval of one year, Table 2.
Commentary
IPD is particularly frequent in chronically ill children and adults. In Denmark every fourth child with invasive pneumococcal disease has an underlying disease, and corresponding figures have been found in other Western countries. In the USA, it was established that more than half of the observed IPD cases occur in persons with an underlying disease. The use of pneumococcal conjugate vaccine in the Danish Childhood Vaccination Programme has reduced the incidence of IPD in children below the age of two years from 54 to 12/105, EPI-NEWS 21/12. Furthermore, it has been demonstrated that conjugate pneumococcal vaccine has an indirect effect in adults aged 50-64 years and 65+ years in the form of a statistically significant reduction in IPD incidence of app. 10% in both of the above-mentioned groups.
It is essential to evaluate and assess the effect of this programme both with regard to IPD incidence and serotype distribution among patients who are at an increased risk of IPD. Furthermore, an assessment of the effect of repeated PPV23 vaccination on IPD incidence and antibody levels over time has been planned.
(P. Valentiner-Branth, L.K. Knudsen, C.H. Suppli, P.H. Andersen, Department of Infectious Disease Epidemiology, T. Dalby, Z.B. Harboe, H. Ingels, S. Hoffmann, H.B. Konradsen, Department of Microbiological Diagnostics and Virology)
Merry Christmas & Happy New Year
Unless special circumstances arise, EPI-NEWS will not be published in week 52/12 and 1/13.
The Department of infectious Disease Epidemiology wishes everyone a merry Christmas and a happy New Year.
Link to previous issues of EPI-NEWS
19 December 2012